Abstract
Peri-prosthetic bone adaptation has usually been predicted using subject-specific finite element analysis in combination with remodelling algorithms and assuming isotropic bone material property. The objective of the study is to develop an orthotropic bone remodelling algorithm for evaluation of peri-prosthetic bone adaptation in the uncemented implanted femur. The simulations considered loading conditions from a variety of daily activities. The orthotropic algorithm was tested on 2D and 3D models of the intact femur for verification of predicted results. The predicted orthotropic directionality, based on principal stress directions, was in agreement with the trabecular orientation in a micro-CT data of proximal femur. The validity of the proposed strain-based algorithm was assessed by comparing the predicted results of the orthotropic model with those of the strain-energy-density-based isotropic formulation. Despite agreement in cortical densities \((R = 0.71)\), the isotropic remodelling algorithm tends to predict relatively higher values around the distal tip of the implant as compared to the orthotropic model. Both formulations predicted 4–8% bone resorption in the proximal femur. A linear regression analysis revealed a significant correlation \((R = 0.99)\) between the stresses and strains on the cortex of the proximal femur, predicted by the isotropic and orthotropic formulations. Despite reasonable agreement in peri-prosthetic bone density distributions, the quantitative differences with isotropic model predictions highlight the combined influences of bone orthotropy and mechanical stimulus in the adaptation process.








Similar content being viewed by others
References
Ahrens J, Geveci B, Law C (2005) Paraview: An end-user tool for large data visualization. Vis Handb 717(8)
Akhavan S, Matthiesen MM, Schulte L, Penoyar T, Kraay MJ, Rimnac CM, Goldberg VM (2006) Clinical and histologic results related to a low-modulus composite total hip replacement stem. JBJS 88(6):1308–1314
Aldinger P, Sabo D, Pritsch M, Thomsen M, Mau H, Ewerbeck V, Breusch S (2003) Pattern of periprosthetic bone remodeling around stable uncemented tapered hip stems: a prospective 84-month follow-up study and a median 156-month cross-sectional study with dxa. Calcif Tissue Int 73(2):115–121
Avval PT, Klika V, Bougherara H (2014) Predicting bone remodeling in response to total hip arthroplasty: computational study using mechanobiochemical model. J Biomech Eng 136(5):051002
Banijamali SMA, Oftadeh R, Nazarian A, Goebel R, Vaziri A, Nayeb-Hashemi H (2015) Effects of different loading patterns on the trabecular bone morphology of the proximal femur using adaptive bone remodeling. J Biomech Eng 137(1)
Beaupré G, Orr T, Carter D (1990a) An approach for time-dependent bone modeling and remodeling–application: A preliminary remodeling simulation. J Orthop Res 8(5):662–670
Beaupré G, Orr T, Carter D (1990b) An approach for time-dependent bone modeling and remodeling–theoretical development. J Orthop Res 8(5):651–661
Bendsoe MP, Sigmund O (2013) Topology optimization: theory, methods, and applications. Springer, Berlin
Bergmann G, Deuretzbacher G, Heller M, Graichen F, Rohlmann A, Strauss J, Duda G (2001) Hip contact forces and gait patterns from routine activities. J Biomech 34(7):859–871
Bitsakos C, Kerner J, Fisher I, Amis AA (2005) The effect of muscle loading on the simulation of bone remodelling in the proximal femur. J Biomech 38(1):133–139
Boyle C, Kim IY (2011) Three-dimensional micro-level computational study of wolff’s law via trabecular bone remodeling in the human proximal femur using design space topology optimization. J Biomech 44(5):935–942
Caouette C, Bureau M, Vendittoli PA, Lavigne M, Nuno N (2012) Anisotropic bone remodeling of a biomimetic metal-on-metal hip resurfacing implant. Med Eng Phys 34(5):559–565
Carter D, Fyhrie DP, Whalen R (1987) Trabecular bone density and loading history: regulation of connective tissue biology by mechanical energy. J Biomech 20(8):785–794
Carter D, Orr T, Fyhrie DP (1989) Relationships between loading history and femoral cancellous bone architecture. J Biomech 22(3):231–244
Chanda S, Dickinson A, Gupta S, Browne M (2015a) Full-field in vitro measurements and in silico predictions of strain shielding in the implanted femur after total hip arthroplasty. Proc Inst Mech Eng H 229(8):549–559
Chanda S, Gupta S, Kumar Pratihar D (2015b) A genetic algorithm based multi-objective shape optimization scheme for cementless femoral implant. J Biomech Eng 137(3)
Colabella L, Cisilino AP, Fachinotti V, Kowalczyk P (2019) Multiscale design of elastic solids with biomimetic cancellous bone cellular microstructures. Struct Multidiscip Optim 60(2):639–661
Cordebois J, Sidoroff F (1982) Damage induced elastic anisotropy. In: Mechanical behavior of anisotropic solids/comportment Méchanique des Solides Anisotropes, Springer, pp 761–774
Cowin SC et al (2001) Bone mechanics handbook. CRC Press, Boca Raton
Cowin SC, Van Buskirk WC (1986) Thermodynamic restrictions on the elastic constants of bone. J Biomech 19(1):85–87
Cuppone M, Seedhom B, Berry E, Ostell A (2004) The longitudinal young’s modulus of cortical bone in the midshaft of human femur and its correlation with ct scanning data. Calcif Tissue Int 74(3):302–309
Dan D, Germann D, Burki H, Hausner P, Kappeler U, Meyer RP, Klaghofer R, Stoll T (2006) Bone loss after total hip arthroplasty. Rheumatol Int 26(9):792–798
Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM (1990) An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans Biomed Eng 37(8):757–767
Doblaré M, Garcıa J (2001) Application of an anisotropic bone-remodelling model based on a damage-repair theory to the analysis of the proximal femur before and after total hip replacement. J Biomech 34(9):1157–1170
Doblaré M, Garcıa J (2002) Anisotropic bone remodelling model based on a continuum damage-repair theory. J Biomech 35(1):1–17
Duda GN, Brand D, Freitag S, Lierse W, Schneider E (1996) Variability of femoral muscle attachments. J Biomech 29(9):1185–1190
Duda GN, Heller M, Albinger J, Schulz O, Schneider E, Claes L (1998) Influence of muscle forces on femoral strain distribution. J Biomech 31(9):841–846
Enns-Bray WS, Owoc JS, Nishiyama KK, Boyd SK (2014) Mapping anisotropy of the proximal femur for enhanced image based finite element analysis. J Biomech 47(13):3272–3278
Fernandes P, Guedes JM, Rodrigues H (1999) Topology optimization of three-dimensional linear elastic structures with a constraint on ”perimeter”. Comput Struct 73(6):583–594
Frost HM (1964) The laws of bone structure. springfield il. Charles C Thomas
Frost HM (2003) Bone’s mechanostat: a 2003 update. Anat Rec Part A Discov Mol Cell Evol Biol Offi Publ Am Assoc Anat 275(2):1081–1101
Garcia J, Martinez M, Doblaré M (2001) An anisotropic internal-external bone adaptation model based on a combination of cao and continuum damage mechanics technologies. Comput Methods Biomech Biomed Engin 4(4):355–377
Garcıa J, Doblaré M, Cegonino J (2002) Bone remodelling simulation: a tool for implant design. Comput Mater Sci 25(1):100–114
Gasbarra E, Iundusi R, Perrone FL, Saturnino L, Tarantino U (2015) Densitometric evaluation of bone remodelling around trabecular metal primary stem: a 24-month follow-up. Aging Clin Exp Res 27(1):69–75
George D, Allena R, Remond Y (2018) A multiphysics stimulus for continuum mechanics bone remodeling. Math Mech Complex Syst 6(4):307–319
Geraldes DM, Phillips A (2014) A comparative study of orthotropic and isotropic bone adaptation in the femur. Int J Number Meth Bio 30(9):873–889
Geraldes DM, Modenese L, Phillips AT (2016) Consideration of multiple load cases is critical in modelling orthotropic bone adaptation in the femur. Biomech Model Mechanobiol 15(5):1029–1042
Ghosh R, Gupta S, Dickinson A, Browne M (2013) Experimental validation of numerically predicted strain and micromotion in intact and implanted composite hemi-pelvises. Proc Inst Mech Eng H 227(2):162–174
Giorgio I, Andreaus U, Scerrato D, Dell’Isola F (2016) A visco-poroelastic model of functional adaptation in bones reconstructed with bio-resorbable materials. Biomech Model Mechanobiol 15(5):1325–1343
Giorgio I, Dell’Isola F, Andreaus U, Alzahrani F, Hayat T, Lekszycki T (2019) On mechanically driven biological stimulus for bone remodeling as a diffusive phenomenon. Biomech Model Mechanobiol 18(6):1639–1663
Gruen TA, McNeice GM, Amstutz HC (1979) ”modes of failure” of cemented stem-type femoral components: a radiographic analysis of loosening. Clin Orthop Relat Res 141:17–27
Huiskes R, Weinans H, Grootenboer H, Dalstra M, Fudala B, Slooff T (1987) Adaptive bone-remodeling theory applied to prosthetic-design analysis. J Biomech 20(11):1135–1150
Huiskes R, Ruimerman R, Van Lenthe GH, Janssen JD (2000) Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 405(6787):704
Jacobs CR, Simo JC, Beaupre GS, Carter DR (1997) Adaptive bone remodeling incorporating simultaneous density and anisotropy considerations. J Biomech 30(6):603–613
Judex S, Zernicke RF (2000) High-impact exercise and growing bone: relation between high strain rates and enhanced bone formation. J Appl Physiol 88(6):2183–2191
Kalmey J, Lovejoy CO (2002) Collagen fiber orientation in the femoral necks of apes and humans: do their histological structures reflect differences in locomotor loading? Bone 31(2):327–332
Kerner J, Huiskes R, Van Lenthe G, Weinans H, Van Rietbergen B, Engh C, Amis A (1999) Correlation between pre-operative periprosthetic bone density and post-operative bone loss in tha can be explained by strain-adaptive remodelling. J Biomech 32(7):695–703
Ki T, Adachi T, Tomita Y (2002) Functional adaptation of cancellous bone in human proximal femur predicted by trabecular surface remodeling simulation toward uniform stress state. J Biomech 35(12):1541–1551
Lanyon L (1987) Functional strain in bone tissue as an objective, and controlling stimulus for adaptive bone remodelling. J Biomech 20(11–12):1083–1093
Lengsfeld M, Günther D, Pressel T, Leppek R, Schmitt J, Griss P (2002) Validation data for periprosthetic bone remodelling theories. J Biomech 35(12):1553–1564
Levadnyi I, Awrejcewicz J, Gubaua JE, Pereira JT (2017) Numerical evaluation of bone remodelling and adaptation considering different hip prosthesis designs. Clin Biomech 50:122–129
Marangalou JH, Ito K, van Rietbergen B (2012) A new approach to determine the accuracy of morphology-elasticity relationships in continuum fe analyses of human proximal femur. J Biomech 45(16):2884–2892
Martin RB (1984) Porosity and specific surface of bone. Crit Rev Biomed Eng 10(3):179–222
Mathai B, Gupta S (2019) Numerical predictions of hip joint and muscle forces during daily activities: a comparison of musculoskeletal models. Proc Inst Mech Eng H 233(6):636–647
Mathai B, Gupta S (2020) The influence of loading configurations on numerical evaluation of failure mechanisms in an uncemented femoral prosthesis. Int J Number Meth Bio 36(8):e3353. https://doi.org/10.1002/cnm.3353
McNamara BP, Taylor D, Prendergast PJ (1997) Computer prediction of adaptive bone remodelling around noncemented femoral prostheses: the relationship between damage-based and strain-based algorithms. Med Eng Phys 19(5):454–463
Miller Z, Fuchs MB, Arcan M (2002) Trabecular bone adaptation with an orthotropic material model. J Biomech 35(2):247–256
Morgan EF, Bayraktar HH, Keaveny TM (2003) Trabecular bone modulus-density relationships depend on anatomic site. J Biomech 36(7):897–904
Mukherjee K, Gupta S (2016a) Bone ingrowth around porous-coated acetabular implant: a three-dimensional finite element study using mechanoregulatory algorithm. Biomech Model Mechanobiol 15(2):389–403
Mukherjee K, Gupta S (2016b) The effects of musculoskeletal loading regimes on numerical evaluations of acetabular component. Proc Inst Mech Eng H 230(10):918–929
Mukherjee K, Gupta S (2017) Combined bone ingrowth and remodeling around uncemented acetabular component: a multiscale mechanobiology-based finite element analysis. J Biomech Eng 139(9):091007
Pedersen P (1989) On optimal orientation of orthotropic materials. Structural optimization 1(2):101–106
Phillips A (2009) The femur as a musculo-skeletal construct: a free boundary condition modelling approach. Med Eng Phys 31(6):673–680
Phillips AT, Villette CC, Modenese L (2015) Femoral bone mesoscale structural architecture prediction using musculoskeletal and finite element modelling. Int Biomech 2(1):43–61
Pidaparti R, Turner C (1997) Cancellous bone architecture: advantages of nonorthogonal trabecular alignment under multidirectional joint loading. J Biomech 30(9):979–983
Polgar K, Gill H, Viceconti M, Murray D, O’connor J (2003) Strain distribution within the human femur due to physiological and simplified loading: finite element analysis using the muscle standardized femur model. Proc Inst Mech Eng H 217(3):173–189
Pontzer H, Lieberman DE, Momin E, Devlin MJ, Polk J, Hallgrimsson B, Cooper D (2006) Trabecular bone in the bird knee responds with high sensitivity to changes in load orientation. J Exp Biol 209(1):57–65
Prendergast P (1997) Finite element models in tissue mechanics and orthopaedic implant design. Clin Biomech 12(6):343–366
Rancourt D, Shirazi-Adl A, Drouin G, Paiement G (1990) Friction properties of the interface between porous-surfaced metals and tibial cancellous bone. J Biomed Mater Res 24(11):1503–1519
San Antonio T, Ciaccia M, Müller-Karger C, Casanova E (2012) Orientation of orthotropic material properties in a femur fe model: a method based on the principal stresses directions. Med Eng Phys 34(7):914–919
Sarikanat M, Yildiz H (2011) Determination of bone density distribution in proximal femur by using the 3d orthotropic bone adaptation model. Proc Inst Mech Eng H 225(4):365–375
Schileo E, Taddei F, Malandrino A, Cristofolini L, Viceconti M (2007) Subject-specific finite element models can accurately predict strain levels in long bones. J Biomech 40(13):2982–2989
Shang Y, Bai J, Peng L (2008) The effects of the spatial influence function on orthotropic femur remodelling. Proc Inst Mech Eng H 222(5):601–609
Singh M, Nagrath A, Maini P (1970) Changes in trabecular pattern of the upper end of the femur as an index of osteoporosis. JBJS 52(3):457–467
Skedros JG, Baucom SL (2007) Mathematical analysis of trabecular ‘trajectories’ in apparent trajectorial structures: the unfortunate historical emphasis on the human proximal femur. J Theor Biol 244(1):15–45
Speirs AD, Heller MO, Duda GN, Taylor WR (2007) Physiologically based boundary conditions in finite element modelling. J Biomech 40(10):2318–2323
Taddei F, Pancanti A, Viceconti M (2004) An improved method for the automatic mapping of computed tomography numbers onto finite element models. Med Eng Phys 26(1):61–69
Taddei F, Schileo E, Helgason B, Cristofolini L, Viceconti M (2007) The material mapping strategy influences the accuracy of ct-based finite element models of bones: an evaluation against experimental measurements. Med Eng Phys 29(9):973–979
Taylor M, Prendergast PJ (2015) Four decades of finite element analysis of orthopaedic devices: where are we now and what are the opportunities? J Biomech 48(5):767–778
Taylor W, Roland E, Ploeg H, Hertig D, Klabunde R, Warner M, Hobatho M, Rakotomanana L, Clift S (2002) Determination of orthotropic bone elastic constants using fea and modal analysis. J Biomech 35(6):767–773
ten Broeke RH, Hendrickx RP, Leffers P, Jutten LM, Geesink RG (2012) Randomised trial comparing bone remodelling around two uncemented stems using modified gruen zones. Hip Int 22(1):41–49
ten Broeke RH, Tarala M, Arts JJ, Janssen DW, Verdonschot N, Geesink RG (2014) Improving peri-prosthetic bone adaptation around cementless hip stems: a clinical and finite element study. Med Eng Phys 36(3):345–353
Turner CH, Anne V, Pidaparti RM (1997) A uniform strain criterion for trabecular bone adaptation: do continuum-level strain gradients drive adaptation? J Biomech 30(6):555–563
Van Rietbergen B, Huiskes R, Weinans H, Sumner D, Turner T, Galante J (1993) The mechanism of bone remodeling and resorption around press-fitted tha stems. J Biomech 26(4–5):369–382
van Rietbergen B, Weinans H, Huiskes R, Odgaard A (1995) A new method to determine trabecular bone elastic properties and loading using micromechanical finite-element models. J Biomech 28(1):69–81
Verhulp E, van Rietbergen B, Huiskes R (2006) Comparison of micro-level and continuum-level voxel models of the proximal femur. J Biomech 39(16):2951–2957
Villette CC, Phillips AT (2016) Informing phenomenological structural bone remodelling with a mechanistic poroelastic model. Biomech Model Mechanobiol 15(1):69–82
Ward F (1838) Outlines of human osteology. Henry Renshaw, London
Weinans H, Huiskes R, van Rietbergen B, Sumner D, Turner T, Galante J (1993) Validation of adaptive bone-remodeling analysis to predict bone morphology around noncemented tha. Mechanically induced bone adaptations around orthopaedic implants pp 19–40
Wolff J (1986) The law of bone remodelling. translated by p. maquet and r. furlong. New York, Springer 1(9):8
Yang H, Ma X, Guo T (2010) Some factors that affect the comparison between isotropic and orthotropic inhomogeneous finite element material models of femur. Med Eng Phys 32(6):553–560
Acknowledgements
The authors would like to convey our gratitude to Dr. Joydeep Banerjee Chowdhury, Head of the Department of Orthopaedics in AMRI Hospital, Salt Lake, Kolkata, India, for his clinical suggestions on total hip replacement surgery.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors, hereby, state that with regard to this study there are no financial or personal relationships with other people and organizations.
Appendix 1
Appendix 1
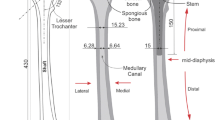
The orthotropic remodelling algorithm was verified using a simplified orthotropic remodelling algorithm applied on a 2D model of proximal femur. The dominant material direction in the computational domain was calculated and Young’s modulus were modified according to the strain stimulus (Sect. 2.2). The results are depicted in Fig. 9.
Orthotropic bone adaptation in 2D FE model of a proximal femur; a FE model with applied loading and boundary conditions, b main functional groups of trabeculae, c predicted material orientation of maximum principal stress: arrows represents dominant material direction groups and are coloured according to Young’s modulus values, d material orientation of minimum principal stress; arrows represents dominant compressive groups and are coloured according to Young’s modulus values e evolution of bone density distributions
Rights and permissions
About this article
Cite this article
Mathai, B., Dhara, S. & Gupta, S. Orthotropic bone remodelling around uncemented femoral implant: a comparison with isotropic formulation. Biomech Model Mechanobiol 20, 1115–1134 (2021). https://doi.org/10.1007/s10237-021-01436-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-021-01436-6