Abstract
Relatively impermeable soil-free substrates (clay-rich outcrops and sediments) exposed at eight abandoned placer gold mines generate alkaline mine drainage through evaporation and minor interaction between water and rock in a semi-arid rain shadow climate. Original mine sluicing a century ago created drainage channels over the land surface that still control the flow of ephemeral surface waters, with localised construction of erosional outwash pans. Ephemeral surface waters and associated evaporitic salts are dominated by halite derived from marine aerosols in rain, yielding circumneutral pH and electrical conductivity (EC) values locally exceeding 50 mS/cm. Weakly altered schist basement rocks and Miocene mudstone exposed at the surface contain abundant calcite, and surface waters are supersaturated with respect to Ca-carbonate minerals with a pH of ≈ 8 and an EC of ≈ 1 mS/cm. Water interaction with albite increases the dissolved Na/Cl molar ratio to > 1, and evaporative formation of Na-carbonate precipitates can raise the pH to > 10. Pyrite oxidation does not offset the alkaline pH in any of these processes, although dissolved sulfate can rise to high levels (> 2000 mg/L; EC ≈ 5 mS/cm), forming evaporative sulfate minerals. Ephemeral waters drain down outcrops and the relatively impermeable erosional pans, leaving salt encrustations with variable mineralogy and associated waters with pH and EC values that are partially controlled by rates of dissolution and reprecipitation of the minerals in the salts. The saline alkaline chemistry of the pans excludes most vegetation and has allowed development of salt-tolerant ecosystems with rare endemic halophytic plants. Maintenance of soil-free halophytic surfaces has potential applications in dryland mine sites around the world, especially as climate change enhances and extends aridity.
摘 要
西兰南部处于半干旱-多云气候条件,8个废弃砂金矿区暴露的相对不透水的无土基质(富含粘土的露头和沉积物)通过蒸发和水-岩微弱相互作用产生碱性矿井水。一个世纪前的矿井开闸工程在地表上形成的排水渠道仍然控制着短暂的地表水径流,并在局部形成了侵蚀性冲刷盆地。降雨过程中形成的暂时性地表水及相关的蒸发盐主要由雨中海洋气溶胶产生的岩盐组成,pH值接近中性,局部电导率(EC)超过50 mS/cm。地表出露的弱变质片岩基岩和中新世泥岩中均含有丰富的方解石,并且地表水中的碳酸钙矿物过饱和,pH值约为8,EC值约为1 mS/cm。水与钠长石的相互作用使溶解的Na/Cl摩尔比提高至>1,蒸发形成碳酸钠沉淀使pH值提高至>10。尽管溶解的硫酸盐可以提升到一个很高的水平(>2000 mg/L;EC约5 mS/cm),并形成蒸发性硫酸盐矿物,但在这些过程中,黄铁矿的氧化不会抵消碱性pH值。暂时性矿井水从露头和相对不透水的侵蚀盆地中溢出,留下具有可变矿物学特征的盐结壳和相关水体,水体的pH值和EC值受到盐类中矿物溶解和再沉淀速率的部分控制。盆地的盐碱化学特性排斥了大部分植被,并形成了一种具有罕见特有盐生植物的耐盐生态系统。因此,在世界各气候干旱的矿区,尤其是在气候变化可能会加剧和扩大干旱的情况下,维持地表的无土壤盐生植物具有潜在的应用价值。
Zusammenfassung
In relativ geringdurchlässigen, bodenfreien Ausgangsgesteinen (tonreiche Aufschlüsse, Sedimente), die in acht stillgelegten Seifengoldabbauen unter semiariden Klimabedingungen freigelegt worden sind, wird alkalisches Grubenwasser durch Verdunstungsprozesse sowie untergeordnet auch durch Wasser-Gesteins-Wechselwirkungen gebildet. Die seit einem Jahrhundert erfolgenden bergbaubedingten Auswaschungsprozesse schufen Entwässerungskanäle an der Landoberfläche, welche immer noch den temporär auftretenden Oberflächenwasserabflusss kontrollieren. Lokal entstanden erosionsbedingte Auswaschungsbecken. Das temporär vorhandene Oberflächenwasser und die entstehenden Evaporitsalze bestehen hauptsächlich aus Halit. Der Niederschlag enthält marine Aerosole. Das Oberflächenwasser weist mehr oder weniger neutrale pH-Werte sowie elektrische Leitfähigkeiten (EC) von z. T. über 50 mS/cm auf. Die an der Oberfläche verbreiteten, gering alterierten Schiefersteine des Grundgebirges sowie die miozänen Tonsteine enthalten überwiegend Kalzit. Das Oberflächenwasser ist mit pH-Werten von bis zu 8 und EC-Werten von ca. 1 mS/cm im Hinblick auf Ca-Karbonat-Minerale übersättigt. Durch Wechselwirkungen mit Albit erhöht sich das molare Verhältnis von gelöstem Na/Cl auf >1. Die durch Verdunstung gebildeten Na-Karbonat-Ausfällungen können den pH-Wert auf >10 anheben. Die Oxidation von Pyrit gleicht den alkalischen pH-Wert bei keinem dieser Prozesse aus, obwohl die Sulfatgehalte auf Werte > 2000 mg/L (EC ca.5 mS/cm) ansteigen können und sich verdunstungsbedingt Sulfatminerale bilden. Das temporär vorhandene Wasser fließt über die aufgeschlossenen Substrate und die relativ undurchlässigen Erosionspfannen und hinterlässt Salzkrusten mit unterschiedlicher Mineralzusammensetzung. Die pH- und EC-Werte werden teilweise durch die Auflösungs- und Wiederausfällungsraten der Salzminerale gesteuert. Das salzhaltige alkalische Wasser in den beckenförmigen Hohlformen führt dazu, dass sich salztolerante Ökosysteme mit seltenen endemischen halophytischen Pflanzen entwickeln können. Die Aufrechterhaltung bodenfreier halophytischer Oberflächen hat vor dem Hintergrund künftig möglicher längerer klimabedingter Trockenphasen evtl. ein höheres Nutzungs- bzw. Anwendungspotential für Bergwerke in Trockengebieten.
Resumen
Los sustratos sin suelo relativamente impermeables (afloramientos y sedimentos ricos en arcilla) expuestos en ocho minas de oro aluvial abandonadas generan un drenaje de minas alcalino a través de la evaporación y de una pequeña interacción entre el agua y la roca en un clima semiárido de sombra de lluvia. La extracción original de la mina hace un siglo creó canales de drenaje sobre la superficie del terreno que todavía controlan el flujo de las aguas superficiales efímeras, con la construcción localizada de balsas erosivas. Las aguas superficiales efímeras y las sales evaporíticas asociadas están dominadas por la halita derivada de los aerosoles marinos en la lluvia, dando lugar a valores de pH y conductividad eléctrica (CE) circunneutrales que superan localmente los 50 mS/cm. Las rocas basales de esquisto débilmente alteradas y la lodolita del Mioceno expuesta en la superficie contienen abundante calcita y las aguas superficiales están sobresaturadas con respecto a los minerales de Ca-carbonato con un pH de ~8 y una CE de ~1 mS/cm. La interacción del agua con la albita aumenta la relación molar Na/Cl disuelto a valores >1, y la formación evaporativa de precipitados de Na-carbonato puede elevar el pH por encima de 10. La oxidación de la pirita no compensa el pH alcalino en ninguno de estos procesos, aunque el sulfato disuelto puede elevarse a niveles altos (>2000 mg/L; CE ~5 mS/cm), formando minerales de sulfato por evaporación. Las aguas efímeras drenan por los afloramientos y las cubetas erosivas relativamente impermeables, dejando incrustaciones de sal con mineralogía variable y aguas asociadas con valores de pH y CE que están parcialmente controlados por las tasas de disolución y reprecipitación de los minerales de las sales. La química alcalina de las salinas excluye la mayor parte de la vegetación y ha permitido el desarrollo de ecosistemas tolerantes a la sal con raras plantas halófilas endémicas. El mantenimiento de las superficies halófilas libres de suelo tiene aplicaciones potenciales en los sitios mineros de las tierras secas de todo el mundo, especialmente porque el cambio climático puede aumentar y extender la aridez.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Exposed bare rock at mines commonly generates acid mine drainage from pyrite oxidation, and this can have major negative environmental effects (Amos et al. 2015; Dold 2017; Lottermoser 2010; Parbhakar-Fox and Lottermoser 2015). Saline mine discharge waters can also cause negative environmental effects (Panagopoulos and Giannika (2022). Alkaline mine drainage is also common but has fewer negative environmental effects than acid drainage (Kirby et al. 2009; Lottermoser 2010; Plante et al. 2011; Smith et al. 2013). Carbonate-related water–rock reactions associated with alkaline rock drainage are commonly seen as beneficial as they absorb atmospheric CO2 (Hamilton et al. 2020; Harrison et al. 2013; Khalidy and Santos 2021; Manning et al. 2013). Hence, there are potential environmental benefits from leaving some such rocks exposed, rather than covering them with soil during rehabilitation which is the normal practice (Anawar 2015; MacDonald et al. 2015; Tropek et al. 2012; Wong 2003).
Another disadvantage of engineered soil covers is that the end result is typically uniform and may have lower biodiversity values than the original landscape (Frouz 2018; Prach and Hobbs 2008; Sonter et al. 2018, 2020; Tropek et al. 2012, 2013). In contrast, there is evidence that limiting engineered rehabilitation activities, and ensuring that a range of surface environments is exposed, can have better long-term biodiversity outcomes (Doley and Audet 2013; Haigh 2018; Parraga-Aguado et al. 2013; Prach and Hobbs 2008; Tropek et al. 2012, 2013). Even localised acid-generating rocks can yield biodiverse ecosystem recovery (Anawar 2015; Nikolic et al. 2016; Valente et al. 2012). More generally, alkaline and saline mine substrates, both soil and bare rock, can host a range of novel ecosystems (Clemente et al. 2012; Frouz 2018; Manousaki and Kalogerakis 2011; Mendez and Maier 2008; Parraga-Aguado et al. 2013; Tropek et al. 2010). In this study, we quantify hydrogeochemical processes that lead to ephemeral alkaline mine drainage and associated evaporative saline substrates in an area with a semi-arid climate (Fig. 1a–c). This surface water network has produced localised enhanced biodiversity on mined land without addition of a post-mining soil cover. These geochemical processes and associated plant communities have evolved naturally on bare soil-free ground after historic mine sites were abandoned without rehabilitation. The sites we describe are small (hectares), but they are biologically important for their specialist plant species (halophytes) that are present because of the mining activity, rather than despite it.
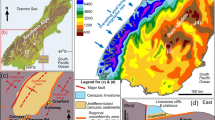
Location and setting of this study in southern New Zealand. a Principal geological features of South Island of New Zealand in relation to this study. b Annual precipitation map of the southern South Island (after NIWA 2020), showing the location of the rain shadow (rainfall areas ≈ 400 mm/year). c Decade record (log scale, millimetres) of daily rainfall and potential evaporation (NIWA 2021) that leads to persistence of mine site salt pans in the rain shadow study area
In our previous work on this topic, we outlined the general principles of this approach to biodiversity-enhanced rehabilitation for acid and circumneutral mine drainage (Craw and Rufaut 2021; Rufaut et al. 2018). We also developed a regional scale understanding of the geochemical processes that lead to formation of alkaline saline substrates above a wide range of rock types (Craw et al. 2022a, b, 2023). This regional scale work evolved from initial studies of two placer mine sites (Druzbicka et al. 2015) that had limited geochemical variability. The present study returns to the mining context and documents a much wider range of mine sites with a much wider range of geochemical variability. In particular, we focus on the geochemical processes that lead to highly saline, circumneutral to highly alkaline (pH ≈ 7 to > 10), surficial environments. We extend and synthesise the previous work on specific historic mine sites (Druzbicka et al. 2015; Law et al. 2016) to a wider range of historic mine sites than previously documented. We also link long-term surface processes at these sites to shorter-term alkaline mine drainage chemical evolution at a nearby active gold mine (Weightman et al. 2020). Our results show that unremediated, exposed mine substrates with ephemeral alkaline and saline runoff can be useful hosts for unique plant communities. In the examples we describe, these communities were not present at the sites before mining, and subsequent land use changes have removed these communities from most surrounding areas. We also provide some preliminary results that show that localised removal of soil can enhance this biodiversity, and that this approach could be scaled up for potential management over larger areas.
General Setting
The Mesozoic Otago Schist belt of southern New Zealand (Fig. 1a) has been a major gold producer for the past 160 years (Craw and MacKenzie 2016; Williams 1974). Most historic gold production (> 8 Moz) was from placer deposits that occur in Cretaceous to Holocene sediments resting on the schist basement (Craw et al. 2016; Williams 1974). However, the modern Macraes Mine (Fig. 1b) is New Zealand’s largest gold mine and has been developed since 1990, first as an opencast and more recently as an underground mine in a large (> 10 Moz) orogenic gold deposit in basement rocks (Craw and MacKenzie 2016). The metasedimentary schist basement has broadly uniform lower greenschist facies metamorphic mineralogy (Table 1), and the Cretaceous-Holocene sediments were derived from the basement and have similar primary mineralogy.
The Otago Schist goldfield lies within a well-defined rain shadow in the lee of the Southern Alps mountains that have been uplifted immediately east of the Alpine Fault tectonic plate boundary (Fig. 1a, b; Chamberlain et al. 1999). Consequently, the goldfield has a cool semi-arid climate in which precipitation (300–600 mm/year) is less than evapotranspiration (> 700 mm/year) and surface waters are readily evaporated to leave precipitated mineral encrustations (Fig. 1b, c, e; NIWA 2021; Craw et al. 2022a, b, 2023; Weightman et al. 2020). Many of these evaporative encrustations and associated ephemeral surface waters are derived from marine aerosols in rain and have compositions reflective of seawater, dominated by NaCl (Craw et al. 2022a, b, 2023). Evaporative encrustations are most prominent on relatively impermeable soil-free surfaces that include clay-rich outcrops and redeposited clay-rich depositional pans. These bare clay-rich soil-free substrates differ from unvegetated bare soils and coarse basement-derived gravels that occur locally in the area and do not host salts or the endemic halophytes. The compositions of ephemeral surface waters (present in occasional heavy rain events) and associated evaporative precipitates (Table 1) on these bare substrates are the principal topics of the present study.
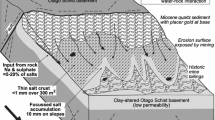
We focus on historic placer gold mine sites in the most arid part of the rain shadow, where some of these abandoned sites have developed unique plant communities over the past century (Figs. 1b, 2a–d). Placer gold was derived from orogenic deposits in the basement rocks and was concentrated at erosional unconformities in the overlying sedimentary sequence (Fig. 2a). The most important unconformity occurs directly on the schist basement where Miocene quartz pebble conglomerates (QPC) host the placers (Fig. 2a, b). Subsequent groundwater passage along the unconformity zone has caused extensive clay alteration of the schist up to 50 m below the contact and some silicification of the overlying QPC (Fig. 2a; Table 1; Chamberlain et al. 1999; Craw et al. 2016). Some alteration above and below the contact was oxidising and produced iron oxyhydroxide while some was reducing and produced authigenic pyrite, especially in association with lignite-bearing sediments associated with the QPCs (Fig. 2a; Table 1; Tostevin et al. 2016).
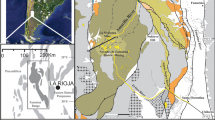
Geological setting for historic placer mine sites in this study. a Sketch of cross section showing the settings of placer mines. b Geological map showing the locations of placer mines in relation to principal geological features in the rain shadow. B Blackmans, O Omeo, CG Conroys Gully, CR Chapman Road, FT Flat Top Hill, TH Tucker Hill, M Manorburn, S Springvale, R current reserves. c Example of partial halophyte cover (salt grasses) on saline outcrop (top) and associated saline pan (bottom) formed after sluicing of clay-altered schist unconformity
Far-field deformation associated with the Alpine Fault and rise of the Southern Alps during the Pliocene and Pleistocene has caused regional-scale folding, uplift, and partial erosion of the Miocene unconformity zone (Fig. 2b; Craw et al. 2022a). Gold has been recycled into Pleistocene sediments during this uplift, and one mine site discussed in this study was developed at the unconformity between Pleistocene gravels and underlying Miocene lacustrine mudstone (Fig. 2a, b). The tectonic uplift and erosion has been on-going, and further recycling of gold has occurred into late Pleistocene and Holocene sediments (Fig. 2a). At the sites discussed in this study, this latter stage is reflected in localised gold redistribution into a surficial veneer of locally-derived gravels, sands and silts (Fig. 2a).
Methods
This paper includes a synthesis of general observations and data from a wide range of natural and anthropogenic sites within New Zealand’s rain shadow, with details recorded by Craw et al. (2022a, b, 2023). Climate information was obtained from NIWA (2021) and ORC (2021). We have distilled these general observations and focused here on eight historic placer mine sites where mining was important for providing suitable saline substrates for halophyte colonisation (Figs. 2, 3, 4). Halophytes are rare at the Manorburn site and the Omeo site does not currently host known halophyte communities on bare saline substrates, although a detailed botanical survey has not been done. Parts of several of these sites are located in formal reserves to preserve these unique plant communities, and our observations were primarily non-destructive because only limited sampling was permitted.
Principal structural and stratigraphic features of historic placer mine sites. a Remnant sluiced cliff of Pleistocene gravel rests unconformably on Miocene mudstone at Springvale site. b Sluiced basement unconformity surface at Conroys Gully; c Remnant QPC channel at Chapman Road. d Coarse gold from Chapman Road site. e Outwash pans formed from sluicing of QPC and gravel veneer on basement unconformities at Flat Top Hill site. f Fine gold particles from Flat Hill site
Bare alkaline saline substrates with halophytes at sluiced mine sites. a, b Chapman Road basement unconformity showing salt deposits, halophytes, and desiccation cracks. c, d Blackmans basement unconformity showing surface development. e–g Springvale mudstone unconformity, where recent rain events had redistributed fine sediments and salts on pan surfaces
Electrical conductivity (EC) and pH have proven to be the most useful parameters for general characterisation of water compositions and saline substrates. The pH and EC of saline substrates were obtained in the field from slurries made with 20 mL of solids and 30 mL of added distilled water. This 2:3 ratio of substrate and water was determined by experience to be the best for ensuring inclusion of adequate salts to the solution to be measured. These slurries required several minutes of stirring to ensure complete disaggregation of the clay-rich materials, and this stirring was found to be the most important preparation stage. The pH and EC of the resultant slurries were measured with a portable Oakton PC450 meter with separate pH and EC probes, which were calibrated in the laboratory (22 °C) with manufacturer-supplied buffer solutions (pH 4.7 and 10; EC 1 mS/cm). The pH and EC of the mine waters were also determined with the same instrument. Surface waters are rare and ephemeral at the historic placer mine sites, and pH and EC data for this study were collected from only two sites in the winter of 2022 after an abnormally wet month.
Identification of minerals in salt encrustations was done primarily using a scanning electron microscope with an energy dispersive analytical attachment (SEM–EDS). Salt samples were obtained from numerous sites across the rain shadow (Craw et al. 2022a, b, 2023), and these results were compiled, along with those specifically made for this study from the mine sites, into Table 1. For SEM analysis, air-dried samples were attached to aluminium stubs with carbon paint and then coated with carbon vapour as for normal SEM preparation. The SEM–EDS observations were done on a Zeiss Sigma VP (variable pressure) SEM fitted with a HKL INCA Premium Synergy Integrated EDX system at the Otago Micro and Nanoscale Imaging (OMNI), University of Otago. Electron backscatter imaging and elemental compositions were obtained at 15 kV. Spot analyses (≈3 µm) caused the destruction of some hydrated mineral surfaces. The fine grain size (micron scale) and intermixing of different salt mineral species and silicates, combined with the rough and irregularly oriented surfaces, produced only semiquantitative results. Analytical limitations prevented definite establishment of stoichiometric compositions of the minerals but allowed identification of general mineral groups (Table 1).
Some salt minerals were identified by x-ray diffraction (XRD) using smears of salt-bearing substrates on glass slides. However, this approach was less successful than using SEM–EDS because of the multiple salt phases present, delicate and ephemeral samples, and dilution by silicates. Associated clay minerals were identified by XRD using smears of the < 2 µm bulk material (kaolinite), and glycolation of smectite, using the approach of earlier more detailed work by Chamberlain et al. (1999) and references therein.
Flowing surface waters on the relatively impermeable substrates are ephemeral, restricted to occasional rain events, are only rarely pooled, and rapidly evaporated to leave salt encrustations. Hence, we created synthetic solutions by leaching samples of the saline crusts in distilled water as part of previous regional studies (Craw et al. 2022b, 2023). We present some representative analyses in Table 2. Samples were obtained from a range of salt crusts and exposed bare substrates at natural and anthropogenic sites in the rain shadow. The samples were selected by volume (≈ 200 mL) and leached in 2 L plastic bottles filled with distilled water for 1 week before being decanted into clean plastic sample bottles and sent for analysis for major ions at Eurofins, Christchurch, New Zealand, an IANZ-accredited commercial laboratory. Analytical techniques are outlined by Craw et al. (2022b, 2023) and are available on the company’s website. We have included observations and water data from the nearby Macraes Mine (Fig. 1b; Table 2; Weightman et al. 2020) for comparison of mine water geochemical evolution processes and formation of evaporative minerals. At the Macraes site, precipitates of aragonite, gypsum, epsomite, and/or bloedite can form from alkaline mine waters (Weightman et al. 2020). In addition, we include some shallow groundwater quality data from the schist basement in the rain shadow (Dunstan Range, Fig. 2b; Table 2; Craw and Kerr 2017) for background comparison. Precipitates of calcite are common from these waters.
Evaporative mineral identifications (Table 1) and their geochemical relationships were augmented by geochemical modelling with the software suite, The Geochemists Workbench® (www.gwb.com), using the Harvie–Moller–Weare activity model for the concentrated evaporation waters. The program REACT was used to model geochemical evolution of mine waters and precipitation of principal minerals during progressive evaporation without mineral redissolution, and all model runs assumed equilibrium with atmospheric CO2. Modelling of contributions to solution EC by ions of differing valency, especially monovalent ions, was done using empirically derived equations summarised by Sposito (2016). Modelling of solution chemistry typically relies on assumptions of chemical equilibrium, which is unlikely in the complex low-temperature situations described in this study. Nevertheless, equilibrium modelling provides some indications of the general chemical trends. The most prominent evidence for disequilibrium in our study is the distinct absence of the mineral dolomite from all evaporative salt encrustations, despite geochemical predictions by Geochemists Workbench modelling. Dolomite is notorious for failing to precipitate in such situations (Arvidson and MacKenzie 1999) and we have therefore suppressed or ignored dolomite in all our modelling.
Results
Description of Placer Mine Sites and Halophytes
The specialist salt-tolerant plants (halophytes) that are linked to this study have become established on bare clay-rich substrates associated with historic placer mining (Fig. 2c). The mining occurred in the late nineteenth century and/or early twentieth century (specific records are poor), with some minor local reworking in the 1930s. The principal mining method involved hydraulic sluicing the gold-bearing sediments with water from a high-pressure hose. This technique, widely introduced in the nineteenth century, mobilised large volumes of gravel from outcrops to pass through gold-saving systems and then be discharged farther downstream as tailings (Davies et al. 2020; Hearn 2013). The water flow rates and gradients were sufficient to remove almost all gravel from the sites, leaving only clay-rich residues (Fig. 2c). The richest gold-bearing sediments, and therefore the principal targets of the historic mining, were channels originally incised into the unconformities (Fig. 3a, c, e). Further, site engineering and eroding water flow formed so-called sludge channels in soft rocks underlying the gold-bearing unconformities, aiding removal of coarse debris (Fig. 3a–e). Gold-extraction techniques were not especially efficient; coarse gold particles (> 1 mm; Fig. 3d) were readily saved while much of the smaller gold (< 0.1 mm; Fig. 3f) was lost to tailings in the discharging slurry. Most sluicing channels have persisted to the present day and these control the discharge of ephemeral alkaline mine waters.
After mining ceased, no rehabilitation occurred and the sites were abandoned. However, pastoral grazing occurred over most sites to some extent, and introduced rabbits have affected the surfaces by grazing and burrowing. A century after mining, these sites still retain the surface topography of the mining era, although soft clay-rich material is still being eroded in heavy rain events (Fig. 4a–g). The Tucker Hill site (Figs. 2b, 5a–c, 6a, b) was crossed in 1906 by a railway line after mining had ceased, and culverts now partially control ephemeral runoff from basement and late Pleistocene-Holocene sediments down the sluicing channels.
Tucker Hill mining area, showing where the gold-bearing unconformity beneath Late Pleistocene gravel veneer has been sluiced. a Most intensely altered schist with abundant surface salt and scattered halophytes, especially on erosional pans. b Remnant of less-altered schist in a clay-altered outcrop, with abundant salts on clay-rich surfaces. c Close-up view of salts accumulated on an eroded pan beneath an outcrop of less-altered schist, showing small-scale variations in EC and pH
Tucker Hill mining area above the site in Fig. 5, showing differential basement alteration and surface EC and pH variations. a General view of differential basement alteration, with softer altered schist preferentially removed by sluicing. b Weakly altered schist outcrop with high-pH salts accumulated on bare silty substrate beneath overhang. c SEM backscatter image of Na-carbonate (NaCarb) and Na-sulfate (NaS) from b. d SEM backscatter image of halite (light grey) coating silicates (dark grey–black) on silty substrate in b. Musc muscovite
Most of the sites examined in this study were formed on the Miocene unconformity, where gold-bearing QPCs and/or late Pleistocene-Holocene erosional residues were stripped off to expose the underlying clay-altered schist basement (Figs. 2a, 3b, c, e, 4a–d, 5a, 6a). These sites consist of remnants of clay-rich altered schist outcrops and clay-rich erosional debris (Figs. 4a–d, 5a–c, 6a, b). The clay-rich debris, which also contains quartz pebbles from QPC (rounded) and from schist (angular), forms erosional pans that partially cover outcrops and were redeposited downslope (Figs. 2c, 3b, e, 4a–d, 5b, c). Miocene mudstone exposed by removal of Pleistocene gravel has formed clay-rich outcrops similar to those of the clay-altered schist but without the quartz pebbles. Erosional pans derived from the mudstone are uniformly clay-rich and smooth (Figs. 3a, 4e–g).
The clay-rich pans at all sites are relatively impermeable to surface water penetration and as a result, they develop evaporative salt encrustations (Figs. 4a–g, 5a–c, 6a–d), the geochemical nature of which is a principal topic of this study (below). This salt development on bare substrates has in turn prevented incursion of most plants since mining ceased, although increasing weed infestation is occurring (Figs. 3a, b, c, e, 4c, 5a, 6a). However, the saline substrates have been colonised by halophytes that are tolerant of the extreme geochemical conditions, and they form mats with low-growing species (< 5 cm high; Figs. 2c, 4b, d, f, g; Druzbicka et al. 2015). These halophytes form part of unique endemic inland salt-tolerant ecosystems and parts of five of the mining areas in this study have been formally reserved for this reason (Fig. 2b). The most abundant and widespread of these halophytes, the herb Atriplex buchananii, also occurs in saline settings along the nearby coast, although it is rare in that setting. The salt grass Puccinellia raroflorens (Figs. 2c, 4b) is also widespread in most of these mine sites, but has extremely limited occurrences on the coast (Edgar 1996). The herb Lepidium kirkii is found only in these inland saline sites, and some of the mine sites are important localities where this rare plant is preserved (e.g. Fig. 4g).
Alkaline Salt Pans and Ephemeral Water Runoff
Impermeable substrates exposed in the historic placer mines, which include both outcrop surfaces and redeposited sedimentary pans, develop dry firm surficial crusts (cm scale) with desiccation cracks (Fig. 4b, f, g). These substrates consist of water-sorted schist-derived silicates (Table 1) and, in particular, variably degraded muscovite that has been oriented by ephemeral surface water flow. On steep outcrops, these crusts are friable and readily disintegrate, but outwash pans formed from redeposition of these fine silicates are typically smooth, gently sloping surfaces that coat flat outcrops and the channel bottoms, and these are the principal locations for evaporative mineral accumulation (Figs. 4a–g, 5a–c, 6b–d). Evaporative salts also contribute to cementation of the dry crusts, thereby enhancing the impermeability to surficial ephemeral water flow. Additional salts form on pan surfaces and within the crusts from capillary rise and evaporation of subsurface moisture. The crust-covered pans are the principal sites for halophyte colonisation, as these pans have the most extremely saline surface chemistry.
Evaporative salts on pans are irregularly distributed in time and space, as they are affected by ephemeral surface water runoff in rain events, and seasonal changes in temperature, humidity, and evapotranspiration rates. Rain events in the area are typically light (1–10 mm/day; Fig. 1c) and water runoff is minor, with most water sinking into desiccation cracks on impermeable outcrop and pan surfaces (Fig. 4b, f, g) or soaking into more permeable substrates adjacent to saline pans (Fig. 5c, d). Occasional heavy rain events (up to 100 mm/day; Fig. 1c) can mobilise fine sediment and redistribute it downslope, controlled by the original sluicing channels (Figs. 3a–c, 4a–g, 5a). These events are responsible for removing fine sediment from the surficial zone of clay-altered basement sites, leaving an armour (cm scale) of coarser quartz debris (Figs. 4b, d, 5c). Redeposition of fine sediment on saline pans occurs at the mm to cm scales during these events (Fig. 4g). At the same time, salt encrustations are at least partially redissolved and redistributed. All these surface water processes are short-term (hours to days) and exposed surfaces dry rapidly afterwards, with renewal of salt encrustations. A rare period of frequent heavy rains in the winter of 2022 caused localised and temporary pooling of runoff water in channel floors at two mine sites, Chapman Road and Springvale.
Evaporative Encrustations and Climate
The mineralogy of salt encrustations is strongly affected by climate and weather, especially temperature and relative humidity that affect degrees of hydration of various minerals and solid phases (Table 1; e.g.: Flatt 2002; Jagniecki et al. 2015). Mineral hydration changes daily and seasonally within the climatic ranges of the area. Hence, we categorise the observed minerals into general types, rather than specific identities (Table 1). Average evapotranspiration for the area ranges from > 5 mm/day in summer to < 0.5 mm/day in winter (Fig. 1c), and abundance of salt crusts varies seasonally accordingly. Rain events are broadly uniformly distributed throughout the year with no specific wet and dry seasons (Fig. 1c), so salt encrustations are temporarily depleted and re-established on a time scale of days in all seasons. Most sampling of salts for this study was done in summer when daily temperatures ranged from 15 to 35 °C and relative humidity was low (commonly < 50%). Daily winter temperatures, in contrast, typically ranged from − 5 to + 12 °C, relative humidity was commonly 70–90%, and salt encrustations were less abundant.
Salt deposits at the placer mine sites occur as a combination of thin (mm-cm scale) surface efflorescences and cements in pore spaces and desiccation cracks immediately (≈ 1 cm) below the surface of pans and outcrops. This combination contributes to the formation of firm salty surface crusts. Most of these salt deposits contain at least some halite, as indicated by taste tests in the field and SEM observations, and many encrustations are dominated by halite (Figs. 5c, 6d). Most also contain some detectable Ca and Mg carbonate minerals, as indicated by effervescence in dilute acid and SEM observations, and calcite is particularly abundant at the Springvale mudstone site (Fig. 4e–g). Na-carbonate minerals (Table 1) are common salt constituents as irregular inhomogeneous masses and as fine-grained (µm scale) fibrous crystals (Fig. 6b–d). Na-carbonate minerals typically occur in close association with divalent carbonates (Fig. 6b), and can be accompanied by Al-oxyhydroxides. Na-sulfates (Fig. 6c) and divalent sulfate minerals are also common, with Ca-sulfates most abundant at basement unconformity sites where pyrite has been oxidised in clay-altered basement and overlying QPC remnants. Bloedite is common at these latter sites as well.
The EC and pH of Mine Substrates
Mine sites on the clay-altered basement unconformity have a wide range of EC (< 0.1 to > 10 mS/cm) and pH (< 7 to > 10) on bare substrates, which is a typical range for the rain shadow in general (Figs. 4a–d, 5a–c, 6a, b, 7a–f). Higher EC and moderate-high pH saline substrates have developed in association with impermeable materials, either intensely clay-altered schist or weakly altered schist (Figs. 5a–c, 6a, b). In contrast, the lowest EC and pH values have developed where only moderate alteration has provided relatively permeable substrates (Figs. 5b, c, 6a, b). Both EC and pH can vary widely on the metre scale on pans at most sites, reflecting differential salt distribution and redistribution during ephemeral surface water flow. Halophytes generally persist where EC > 1 mS/cm. Steep gradients in both EC and pH occur near the margins of pans, where some soil development and associated weed incursions have occurred (Fig. 4c, d). The weeds are established in soil veneers with lower pH and low EC (< < 1 mS/cm; Figs. 4c, d, 5b, 6a, 7b), but saline substrates persist beneath these soil veneers and continue to contribute salts to the pans via ephemeral water flow at the base of the soil layers.
Variability in EC and pH on bare alkaline saline substrates. a General EC and pH data from bare substrates across the rain shadow, with data from Tucker Hill gold mine (Figs. 5, 6) for comparison. b Plot of surface and subsurface solid data from Chapman Road gold mining area, with winter surface water data. c Map of the mining area at the Chapman Road basement unconformity site, with sluicing drainage and distribution of EC and pH data. d Variations in EC and pH of winter ephemeral water (as in b) in and near the main sluicing channel through Chapman reserve site in c. e EC and pH plot for five mine sites on the basement unconformity. f EC and pH data for surface and subsurface solids at mudstone placer mine site at Springvale, with a winter runoff water pool measurement
Wide variations in EC and pH occur in a small area (ha scale) at the Tucker Hill site, where sluicing channels have exposed differential basement unconformity alteration (Figs. 5a–c, 6a, b, 7a). The EC and pH ranges in this small area at the Tucker Hill mine site are similar to those of the whole set of natural and anthropogenic sites examined in the rain shadow (Fig. 7a). Similar wide variations are also seen across the largest single mining area, in surface solids and in rare surface waters (Chapman Road; Figs. 2b, 7b–d), although there were less variations at shallow depths at this site (Fig. 6b). The winter ephemeral waters at the Chapman Road reserve site had a generally consistent pH near 8 in and near the main sluicing channel, while EC varied widely as local surface runoff contributed dissolved salts from marginal slopes (Fig. 7c, d).
The pH of bare saline pans at the mudstone unconformity has a relatively restricted range, near pH 7–8 at the surface and at depth, and the one surface water sample had a pH of 6.5 (Fig. 7f). Outcrops above the pans also have a pH near 8 and contain veinlets and surface films of calcite. The EC at this site shows wide variations, similar to those at the basement unconformity (Fig. 7a–e), but halophytes occur on pans where EC > 1 mS/cm. As for the basement unconformity, weed-bearing soils adjacent to the halophytic mudstone-derived pans generally have a lower pH and distinctly lower EC (Fig. 7f). The relatively narrow range of pH at this mudstone site contrasts with the very wide range of pH observations obtained at natural and anthropogenic bare saline surfaces on a range of geological substrates (Miocene–Holocene) in the general area (Fig. 7a–e). However, the range of EC values is similar across all these substrates (Fig. 7a–f).
Ephemeral Water Geochemistry
Leachate solutions derived from many of the salt pans across the rain shadow are characterised by Na and Cl with a molar ratio near 1:1, reflecting the marine aerosol contribution to the saline surfaces and the dissolution of halite (Fig. 8a; Table 2). However, some leachates have relatively elevated Na concentrations resulting from geochemical interactions with the underlying rock: schist basement and schist-derived sediments (Figs. 6a–d, 8a; Table 2). The salt components of these leachate samples contain Na-carbonates and Na-sulfate minerals in addition to NaCl (Figs. 6b–d, 8a; Table 1).
Chemical compositions of waters relevant to this study. a Cl vs. Na plot for leachates from alkaline saline substrates across the rain shadow (Table 2; Craw et al. 2022b, 2023), with principal evaporite minerals. b Time series for waters discharging from fresh waste rock at the Macraes Mine (cf Fig. 1b; Weightman et al. 2020), with principal rock alteration reactions indicated. c Relationship between dissolved Na and EC for leachates in a, and estimated Na range based on maximum observed field EC for halite-rich evaporites. The relationship between NaCl and EC is based on the equation for monovalent ions in Sposito (2016). d Relationships between divalent ions and EC inferred from Macraes waste rock water sulfate data (Weightman et al. 2020) and carbonate-saturated schist basement groundwater from Dunstan Range (Fig. 2b; Craw and Kerr 2017)
Albite is the principal Na-bearing mineral in the schist basement and basement-derived sediments, along with Na-smectite derived from albite alteration (Table 1; Figs. 6a–d, 8a). Interactions between near-surface (vadose zone) waters and albite or Na-smectite lead to progressive increases in dissolved Na, which causes deviations from the Na/Cl 1:1 molar ratio (Fig. 8a). An example of this albite alteration has been tracked with chemical evolution of waters emanating from fresh schist in waste rock piles at the Macraes Mine at the eastern edge of the rain shadow (Figs. 1b, 8b). The Na/Cl molar ratio progressively increased from ≈1 (dominated by marine aerosols) to > 6 (rock-dominated) over ≈ 10 years (Fig. 8b; Table 2).
The salt pan leachates show a similar wide range of pH values as the field pH measurements (Figs. 7a–f, 8a). Leachates with higher pH values show the greatest deviations from the Na/Cl 1:1 molar ratio, and are derived from salts that contain components of Na-carbonate minerals (Figs. 6b–d, 8a; Table 1). Leachate EC values also show a wide range, similar to but less than that seen in field measurements at the mine sites (Figs. 7a–f, 8c). The highest EC values, locally > 50 mS/cm, reflect localised dominance of surface halite (Figs. 5a–c, 6b, 7a, 8c). Larger leachate samples (200 mL) contain more silicate material than the field EC samples (20 mL), thereby diluting the halite content and lowering the observed EC.
Dissolution of salt pan evaporative sulfate minerals such as gypsum, bloedite, and Na-sulfates has only minor effects on EC compared to that of halite (Fig. 8c, d), and no observed effect on pH. For example, very high dissolved sulfate concentrations (up to 3000 mg/L) emanate from the pyrite-bearing Macraes Mine waste rock piles (Table 2). These waters locally precipitate evaporative sulfate minerals, mainly gypsum, epsomite, and/or bloedite, with locally elevated Mg derived from chlorite dissolution (Fig. 8b; Tables 1, 2). The waste rock waters have EC values of only < 5 mS/cm, substantially below the values that can arise from dissolution of halite crusts on salt pans (Fig. 8c, d).
Dissolution of carbonate minerals such as calcite contributes little to the observed EC values, as indicated for example by groundwaters that are essentially saturated with calcite and have EC values < 1 mS/cm (Fig. 8d). However, dissolution of calcite maintains a pH near 8 in the Macraes waste rock waters despite the abundant pyrite dissolution that was also occurring, with resultant elevated sulfate concentrations (Fig. 8b; Table 2). Similarly, calcite in the mudstone at the Springvale placer mine site contributes to the relatively narrow pH range of the salt pans at that site (Figs. 4e, f, 7f) compared to the other saline sites in the rain shadow (Fig. 7a–d).
Discussion
Modelled Solution Chemistry Variations
The chemistry of ephemeral waters derived from rain containing marine aerosols can be modelled as progressive evaporation of dilute sea water (Fig. 9a). This conceptual model predicts that minor gypsum and then abundant halite are the principal minerals to form, while the pH of the residual water decreases below 7 (Fig. 9a). Observed halite-dominated salt pans in our study have the lowest pH (6–8) as predicted by geochemical modelling (Fig. 8a). These halite-dominated pans also have the highest observed EC values, locally exceeding 50 mS/cm, as predicted by modelling of solutions with high concentrations of monovalent ions (Fig. 8c; Sposito 2016). Solutions with divalent ions have lower maximum EC (< 5 mS/cm; Fig. 8d; Sposito 2016).
Geochemical modelling results (25 °C) of water–mineral relationships relevant to generating alkaline saline surface waters. a Geochemists Workbench model results for evaporation of dilute seawater (equivalent to rain with marine aerosols), showing predicted pH evolution of residual water. b Geochemists Workbench model of evaporation of sodic waters that have interacted with albite (Fig. 7a), showing predicted pH evolution of residual water for differing concentrations of Ca. c Relationships of the principal parameters that control calcite solubility and associated pH in surface waters (after Drever 1997). Macraes Mine waste rock leachate (Fig. 7b; Weightman et al. 2020) are generally highly supersaturated. Salt pan leachates also deviate substantially from calcite equilibrium
Modelled evaporation of rock-exchanged waters that have Na/Cl > 1 but low Ca concentrations predicts precipitation of Na-carbonates and relatively elevated pH (Fig. 9b). This is equivalent to the high-pH salt pans formed where ephemeral waters have interacted with albite from the host rocks or sediments (Fig. 8a). However, increasing dissolution of calcite from the host rocks instead drives the solution pH towards circumneutral values (Fig. 9b). Equilibrium dissolution of calcite involves four parameters: the pH and concentrations of Ca2+, HCO3−, and CO2(g), as depicted in Fig. 9c (after Drever 1997). For solutions in equilibrium with atmospheric CO2(g) and calcite, the pH is typically near 8 on the left side of this plot (Fig. 9c). Some leachates from salt pans fall into this general equilibrium zone, and in particular the mudstone site at Springvale shows abundant evidence of calcite interaction (Fig. 7f). However, the more sodic and high-pH salt pan leachates are clearly out of equilibrium with calcite (Fig. 9c) and are instead partly controlled by the dissolution and precipitation of Na-carbonates (Figs. 8a, 9b).
Evolving waste rock waters at the Macraes Mine display extremes of disequilibrium, with severe supersaturation (log Q/K > 1.3; Weightman et al. 2020) with respect to calcite at pH ≈ 8 (Figs. 8b, 9c). These waste rock waters locally precipitate aragonite and gypsum but remain supersaturated with respect to calcite (Weightman et al. 2020). In addition, as the Mg/Ca and sulfate/alkalinity ratios increased with increasing alteration of chlorite and pyrite (Fig. 8b), epsomite becomes the principal evaporative precipitate mineral (Weightman et al. 2020).
Chemical Evolution of Alkaline Ephemeral Waters
Short-term surface water compositions can be locally alkaline as a result of evaporative precipitation of Na-carbonate minerals (Fig. 9b). However, this local alkalinity is ultimately driven by albite alteration in the associated rocks (Fig. 8a, b). Initial albite alteration occurs via a hydration reaction (Mamonov et al. 2020).
Ultimately, albite alteration leads to formation of kaolinite, as observed in the clay-altered schist basement zone (Craw et al. 2022b, 2023).
These generalised reactions provide the alkaline and sodic context in which the Na-carbonate minerals form from evaporating ephemeral waters (Fig. 6b–d).
The field observations, leachate compositions, and geochemical modelling results outlined above lead to broad generalisations of salt pan evolution and associated predictions of pH and EC variations of saline substrates (Fig. 10a–c). Without interaction between incoming rainwater and rock substrate, the pans become dominated by halite with a circumneutral pH and very high EC (Fig. 10a–c). If subsequent water–rock interaction is dominated by calcite, the pH will be ≈ 8 and EC will be low (< 1 mS/cm), as depicted on the right side of Fig. 10a–c. If water–rock interactions are dominated by albite alteration (Eqs. 1 and 2), the pH can rise above 10 with moderate EC (Fig. 10a–c; left side). Most pans have some combination of all of these processes (Fig. 10a–c). In addition, sulfate minerals will form depending on the nature of water–rock interactions (Fig. 10a).
Summary of chemical and mineral variability on alkaline salt pans. a Principal evolutionary processes, starting with halite-dominated pans (top) and interacting with calcite (right) and/or albite (left). b Typical variations in pH that result from the processes in a. c Typical variations in EC that result from the processes in a. Note that salts remain dominated by halite, and developments of Na and Ca carbonates are subordinate. d Comparison of mineral dissolution rates on alkaline saline pans, contrasting water–rock interactions (left) and rainwater–evaporate interactions (right)
Mineral Dissolution Rate Variability
In addition to the above-inferred variations in chemistry and mineralogy of saline pan formation, the pans are subject to ephemeral water inflow in the contexts of on-going weather and climate effects. Different minerals in the pan environments dissolve at different rates, with rate variations spreading over many orders of magnitude (Fig. 10d; Alkattan et al. 1997; Gustaffson and Puigdomenech 2002; Langmuir 1997; Nasun-Saygili and Okutan 1996; Tang et al. 2018). For example, halite encrustations can be dissolved in minutes during a rain event (Fig. 10d), and thereby NaCl can be moved downslope from one part of a pan to another, moved down through the crust via desiccation cracks, or even completely washed off the pan.
At the other end of the rate spectrum, albite alteration progressively raises the Na/Cl molar ratio to > 1 on substrates that still contain some albite. This includes the fresh schist of the Macraes Mine (Fig. 8b) and variably clay-altered substrates at the placer mines but not the most clay-altered substrates (Fig. 6a–d). The resultant elevated dissolved Na concentration contributes to precipitation of Na-carbonates and Na-sulfates and elevated pH values; this process occurs on a time scale of years (Figs. 8b, 10d). Chlorite alteration occurs at broadly similar rates to those of albite (Figs. 8b, 10d), but chlorite is commonly oxidised and altered during long-term formation of the clay-altered schist substrate (Chamberlain et al. 1999) and is therefore absent from many of the placer mine substrates in this study. Hence, while discharging waters from fresh schist can contain abundant dissolved Mg and form Mg-rich evaporative precipitates (Fig. 8b), Mg-bearing minerals are less common on the placer mine salt pans (Table 1).
Between the extremes of silicate and halite dissolution rates, there is a wide range of mineral dissolution rates that can contribute to differential mobility of chemical components and evaporative minerals on the salt pans. This leads to locally inhomogeneous surface mineralogy and chemistry on daily and seasonal time scales (Fig. 10d).
Enhancing Biodiversity
The mining industry has a reputation for decreasing local biodiversity during its activities, and these impacts are predicted to increase (e.g. Sonter et al. 2018, 2020). These biodiversity effects largely result from activities that affect surface environments, such as modification of forests and animal habitats during mine development (Sonter et al. 2018, 2020). However, modern miners are normally required to rehabilitate mine sites during and after mining activity, and it is this rehabilitation phase that can affect long-term biodiversity values (Anawar 2015; Haigh 2018; Parraga-Aguado et al. 2013; Prach and Hobbs 2008). Engineered rehabilitation can, for example, result in a low-biodiversity monoculture if the same species and processes are used over large areas. Engineered land rehabilitation and surface biological re-establishment are also difficult processes, commonly with unpredictable long-term results (Haigh 2018; Prach and Hobbs 2008; Tropek et al. 2012, 2013). Nevertheless, specifically targeting enhanced biodiversity during engineered reclamation may be more successful (Sobolewski and Sobolewski 2022).
In this study, we show that biodiversity of vegetation at the historic placer mines was enhanced compared to the surrounding areas by development of saline substrates and unique endemic halophyte communities. Similar effects have been noted elsewhere (Clemente et al. 2012; Manousaki and Kalogerakis 2011; Parraga-Aguado et al. 2013). This locally extreme surface chemistry helps to exclude exotic weed species, which are common outside the mine sites, that would otherwise dominate the area and lower the net biodiversity. However, our observations of these sites suggest that adventive species do progressively encroach onto the bare substrates on a time scale of decades (e.g. Figs. 3a, b, e, 4c). This encroachment is partially resisted by dynamic physical processes such as erosion and redeposition of fine sediments during rain events. Apparently, the endemic halophytes tolerate such processes (e.g. Fig. 4e–g).
On-going Maintenance and Climate Change
Ultimate incursion of exotic plant species onto the salt pans appears to be inevitable in the long term (decades; Figs. 3a, b, c, e, 4c, 6a). Incursion of these weed species is particularly pronounced in the floors of the historic sluicing channels where ephemeral water runoff has deposited fine sediments (Figs. 3a, b, c, e, 4c, 5a, 6a). Some of this sediment is washed or blown in from the surrounding area, not just the clay-rich immediate substrates, and this material commonly has lower EC and neutral pH values, permitting weed establishment (Craw et al. 2022a). Hence, the saline sites are not permanent features, although they have already survived on decade to century time scales.
One approach to maintenance of the enhanced biodiversity of these alkaline and saline bare substrates is to periodically remove encroaching weeds and associated soil. We have conducted a pilot study of this approach at the Chapman Road placer mine site (Figs. 7c, 11a–c). This study was of limited extent because soil and weeds were removed by hand, but a small area of clay-rich altered schist substrate was left exposed (Fig. 11a). The experiment was remarkably successful in enhancing the distribution of halophytes on alkaline saline substrate in only one year (Fig. 11b), and after two years, the surface was almost completely covered in halophytes (e.g. Figure 11c). In addition, as the redeveloped substrate evolved over two years, clay compaction and local redistribution allowed development of a quartz pebble armour, which helped exclude weeds (Fig. 11c). Additional and larger-scale mechanical and engineered soil and weed removal periodically from sites such as this may be a viable way of maintaining the distinct halophyte ecosystems.
Pilot substrate redevelopment study at Chapman Road historic mining area (Fig. 7c). a Photograph of part of the study area at time of substrate redevelopment, with red dashed line showing the edge of redeveloped area, as indicated also in b. b Map of the study site after 13 months, showing areas of successful colonisation of bare substrates by halophytes within the red dashed line bounding the redeveloped area. c Close-up view of redeveloped surface in December 2020, showing that halophytes (Atriplex buchananii) were already well-established (site shown in b), with a newly-developed quartz pebble armour (white, lower left) on clay substrate
The salinity of the pans is controlled by the semi-arid climate of the area, which is characterised by strongly seasonal evaporation but generally uniformly distributed rainfall with no well-defined wet season (Fig. 1c). Climate change modelling for the area (MfE 2018) predicts rising temperatures and associated enhancement of the rain shadow effect with increased frequency of the warm, dry westerly winds across the mountains that currently help create the rain shadow (Fig. 1b). Consequently, climatic conditions in southern New Zealand are likely to become more favourable for these sites of unique biodiversity. On a global scale, climate change is predicted to enhance aridity over large areas, with an increase in dryland area of > 10% worldwide (Huang et al. 2015). The increased aridity may have negative effects on some biological aspects and facilitate soil erosion (Berdugo et al. 2020; Huang et al. 2015). However, it is also likely to enhance and extend the development of halophytic habitats of the type described in this study. Hence, the biodiverse sites described here may have implications and applications for rehabilitation activities at other dryland mine sites around the world. However, some drylands have a well-defined wet season (e.g. monsoon) that may hinder the longevity of saline substrates, unlike the more distributed rainfall pattern (Fig. 1c) at the sites we have studied.
Conclusions
Historic placer gold mining in the semi-arid rain shadow of the southern South Island, New Zealand, exhumed low-permeability clay-rich substrates beneath the gold-bearing gravels, and these substrates are still exposed a century later. Mining involved sluicing with water, and this process created channels within the underlying substrates. Sluicing was effective at removing most of the gravel, leaving only clay-rich residues as erosional pans. Post-mining ephemeral drainage from the sites has enhanced the development of erosional pans, and this process still continues in periodic rain events. The mine sites still consist of hectare-scale soil-free areas of outcrops and pans, although some marginal encroachment by soil and weeds is occurring. Substrates at seven of the eight mining areas described in this study consist of variably clay-altered metasedimentary schist basement and associated debris, while one site is underlain by Miocene mudstone derived from that basement.
Potential evaporation at the sites exceeds incoming rainfall, so surface runoff is temporary during rain events, and evaporation of water from the surface and immediate subsurface leads to formation of salt encrustations. Ephemeral waters drain down outcrops and the relatively impermeable erosional pans, leaving the salt encrustations with variable mineralogy, pH, and EC that are partially controlled by rates of dissolution and reprecipitation of the salt minerals (Fig. 10d). Rain contains minor amounts of marine aerosols, leading to the precipitation of salts dominated by halite. Surfaces with halite encrustations typically have circumneutral pH and EC values that locally exceeds 50 mS/cm (Fig. 10a–c).
Weakly altered schist basement and Miocene mudstone contain abundant calcite, and surface waters that have interacted with these exposed substrates are supersaturated with respect to Ca-carbonate minerals with a pH ≈ 8 and an EC of ≈ 1 mS/cm, as well as with halite on evaporation (Fig. 10a–c). Water interaction with albite in the bare substrates increases the dissolved Na/Cl molar ratio from ≈ 1 in the incoming rain to produce ephemeral Na-rich waters (Fig. 10a–c). Evaporative formation of Na-carbonate precipitates from these waters can raise the pH to > 10 (Fig. 10a–c). Localised acidification during oxidation of pyrite does not offset the alkaline pH in any of these processes, although the resultant dissolved sulfate concentrations can rise to high levels (> 2000 mg/L; EC ≈ 5 mS/cm). Evaporative sulfate minerals are common, including gypsum and a range of Na-bearing sulfates that form from the sodic waters (Fig. 10a–c). The distribution of evaporative minerals at the sites (Fig. 10d) changes on a wide range of time scales from minutes (rain events) to decades (shallow groundwater).
The saline alkaline chemistry of the pans excludes most vegetation and has allowed salt-tolerant ecosystems with rare endemic halophytic plants to develop. These sites have increased the local biodiversity compared to the surrounding area, and several of the studied sites have been formally reserved for that reason. These halophytic communities are present at the sites because of the mining activity, not despite that activity. The ecosystems have developed naturally on soil-free substrates and provide examples of mine rehabilitation processes in which soil is not necessary. Further, we have shown in a pilot study (Fig. 11a, b) that the enhanced biodiversity of the sites can be preserved and expanded by removal of soil and non-native weeds from the margins of saline pans to expose bare substrate for subsequent colonisation by the rare endemic halophytes. Climate change may enhance these saline substrates in the future.
On a global scale, climate change is predicted to expand areas of aridity in certain areas, and this process may extend the potential suitability of soil-free halophytic substrates in mine rehabilitation activities elsewhere. However, this approach is most effective where annual rainfall is uniformly distributed through time, rather than seasonally. Even then, on-going management intervention, such as periodic soil and non-native vegetation removal, may be needed, in a scaled-up version of our pilot study (Fig. 11).
Data availability
All data related to this study are provided in the paper and in cited references.
References
Alkattan M, Oelkers EH, Dandurand J-L, Schott J (1997) Experimental studies of halite dissolution kinetics, 1. The effect of saturation state and the presence of trace metals. Chem Geol 137:201–219
Amos RT, Blowes DW, Bailey BL, Sego DC, Smith L, Ritchie AIM (2015) Waste rock hydrogeology and geochemistry. Appl Geochem 57:140–156
Anawar HM (2015) Sustainable rehabilitation of mining waste and acid mine drainage using geochemistry, mine type, mineralogy, texture, ore extraction and climate knowledge. J Environ Manag 158:111–121
Arvidson RS, MacKenzie FT (1999) The dolomite problem: control of precipitation kinetics by temperature and saturation state. Am J Sci 299:257–288
Berdugo M, Delgado-Baquerizo M, Soliveres S, Hernández-Clemente R, Zhao Y, Gaitán JJ, Gross N, Saiz H, Maire V, Lehman A, Rillig MC, Sole RV, Maestre FT (2020) Global ecosystem thresholds driven by aridity. Science 367:787–790
Chamberlain CP, Poage MA, Craw D, Reynolds RC (1999) Topographic development of the Southern Alps recorded by the isotopic composition of authigenic clay minerals, South Island, New Zealand. Chem Geol 155:279–294
Clemente R, Walker DJ, Pardo T, Martínez-Fernández D, Bernal MP (2012) The use of a halophytic plant species and organic amendments for the remediation of a trace elements-contaminated soil under semi-arid conditions. J Hazard Mater 223–224:63–71
Craw D, Kerr G (2017) Geochemistry and mineralogy of contrasting supergene gold alteration zones, southern New Zealand. Appl Geochem 85:19–34
Craw D, Rufaut C (2021) Geoecological zonation enhances biodiversity at historic mine sites, southern New Zealand. Minerals 11:181. https://doi.org/10.3390/min11020181
Craw D, Hesson M, Kerr G (2016) Morphological evolution of gold nuggets in proximal sedimentary environments, southern New Zealand. Ore Geol Rev 80:784–799
Craw D, Rufaut C, Pillai D, Read S (2022a) Physical controls on the formation of halophytic saline substrates near Alexandra, Central Otago, New Zealand. N Z J Geol Geophys 65:439–456
Craw D, Rufaut C, Pillai D (2022b) Geological controls on evolution of evaporative precipitates on soil-free substrates and ecosystems, southern New Zealand. Sci Total Environ 849:157792
Craw D, Rufaut C, Pillai D, Kerr G (2023) Geochemical evolution of high-pH sodic salt pans in central Otago, New Zealand. N Z J Geol Geophys 1:1–52. https://doi.org/10.1080/00288306.2022.2076701. (on-line May 2022)
Craw D, MacKenzie D (2016) Macraes gold deposit, New Zealand. SpringerBriefs in world mineral deposits
Davies P, Lawrence S, Turnbull J, Rutherford I, Grove J, Silvester E, Macklin M (2020) Mining modification of river systems: a case study from the Australian gold rush. Geoarchaeology 35:384–399
Dold B (2017) Acid rock drainage prediction: a critical review. J Geochem Explor 172:120–132
Doley D, Audet P (2013) Adopting novel ecosystems as suitable rehabilitation alternatives for former mine sites. Ecol Process 2:22
Drever JI (1997) Geochemistry of natural waters: surface and groundwater environments, 3rd edn. Prentice Hall, Upper Saddle River
Druzbicka J, Rufaut C, Craw D (2015) Evaporative mine water controls on natural revegetation of placer gold mines, southern New Zealand. Mine Water Environ 34:375–387
Edgar E (1996) Puccinellia Parl. (Gramineae: Poeae) in New Zealand, NZ J Botany 34:17–32
Flatt RJ (2002) Salt damage in porous materials: how high supersaturations are generated. J Cryst Growth 242:435–454
Frouz J (2018) Reclamation of forest ecosystems in postmining sites in the Czech Republic and its comparison with unassisted ecosystem development. In: Prasad MNV, Favas P, Maiti SK (eds) Bio-geotechnologies for mine site rehabilitation. Elsevier, Amsterdam, pp 335–346
Gustaffson AB, Puigdomenech I (2002) The effect of pH on chlorite dissolution rates at 25°C. MRS Online Proc Library, vol 757: Symp II, scientific basis for nuclear waste management XXVI , II3.16. https://doi.org/10.1557/PROC-757-II3.16
Haigh M (2018) Building a cradle for nature: a paradigm for environmental reconstruction. In: Prasad MNV, Favas P, Maiti SK (eds) Bio-geotechnologies for mine site rehabilitation. Elsevier, Amsterdam, pp 593–615
Hamilton JL, Wilson SA, Morgan B, Harrison AL, Turvey CC, Paterson DJ, Dipple GM, Southam G (2020) Accelerating mineral carbonation in ultramafic mine tailings via direct CO2 reaction and heap leaching with potential for base metal enrichment and recovery. Econ Geol 115:303–323
Harrison AL, Power IM, Dipple GM (2013) Accelerated carbonation of brucite in mine tailings for carbon sequestration. Environ Sci Technol 47:126–134
Hearn T (2013) Mining the quarry. In: Pawson E, Brooking T (eds) Making a New Land: environmental histories of New Zealand. Otago University Press, Dunedin, pp 106–121
Huang J, Yu H, Guan X, Wang G, Guo R (2015) Accelerated dryland expansion under climate change. Nat Clim Change 6:166–171
Jagniecki E, Jenkins DM, Lowenstein TK (2015) Eocene atmospheric CO2 from the nahcolite proxy. Geology 43:1075–1078
Khalidy R, Santos RM (2021) The fate of atmospheric carbon sequestrated through weathering in mine tailings. Miner Eng 163:106767
Kirby CS, Dennis A, Kahler A (2009) Aeration to degas CO2, increase pH, and increase iron oxidation rates for efficient treatment of net alkaline mine drainage. Appl Geochem 24:1175–1184
Langmuir D (1997) Aqueous environmental geochemistry. Prentice Hall, Upper Saddle River
Law S, Rufaut C, Lilly K, Craw D (2016) Geology, evaporative salt accumulation and geoecology at Springvale historic gold mine, central Otago, New Zealand. N Z J Geol Geophys 59:382–395
Lottermoser B (2010) Mine wastes: characterization, treatment and environmental impacts. Springer, Berlin
Macdonald SE, Landhausser SM, Skousen J, Franklin J, Frouz J, Hall S, Jacobs DF, Quideau S (2015) Forest restoration following surface mining disturbance: challenges and solutions. New for 46:703–732
Mamonov A, Puntervold T, Strand S, Hetland B, Andersen Y, Wealth A, Nadeau PH (2020) Contribution of feldspar minerals to pH during smart water EOR processes in sandstones. Energy Fuels 34:55–64
Manning DAC, Renforth P, Lopez-Capel E, Robertson S, Ghazireh N (2013) Carbonate precipitation in artificial soils produced from basaltic quarry fines and composts: an opportunity for passive carbon sequestration. Int J Greenh Gas Control 17:309–317
Manousaki E, Kalogerakis N (2011) Halophytes present new opportunities in phytoremediation of heavy metals and saline soils. Ind Eng Chem Res 50:656–660
Mendez MO, Maier RM (2008) Phytostabilization of mine tailings in arid and semiarid environments: an emerging remediation technology. Environ Health Perspect 116:278–283
Ministry for the Environment (MfE) (2018) Climate change projections for New Zealand: atmosphere projections based on simulations from the IPCC Fifth Assessment, 2nd edn. Ministry for the Environment, Wellington
Nasun-Saygili G, Okutan H (1996) Mechanism of the dissolution of Turkish trona. Hydrometallurgy 43:317–329
Nikolic N, Bocker R, Nikolic M (2016) Long-term passive restoration following fluvial deposition of sulphidic copper tailings: nature filters out the solutions. Environ Sci Pollut Res 23:13672–13680
NIWA (2021) National Institute and Water and Atmospheric Research websites. https://www.niwa.co.nz/climate; https://www.cliflo.niwa.co.nz
ORC (2021) Otago Regional Council, Monthly evapotranspiration, Lauder site. www.orc.govt.nz/managing-our-environment/land/land-monitoring
Panagopoulos A, Giannika V (2022) Decarbonized and circular brine management/valorization for water & valuable resource recovery via minimal/zero liquid discharge (MLD/ZLD) strategies. J Environ Manag 324:116239
Parbhakar-Fox A, Lottermoser BG (2015) A critical review of acid rock drainage prediction methods and practices. Min Eng 82:107–124
Parraga-Aguado I, Gonzalez-Alcaraz MN, Alvarez-Rogel J, Jimenez-Carceles FJ, Conesa HM (2013) The importance of edaphic niches and pioneer plant species succession for the phytomanagement of mine tailings. Environ Pollut 176:134–143
Plante B, Benzaazoua M, Bussière B (2011) Predicting geochemical behaviour of waste rock with low acid generating potential using laboratory kinetic tests. Mine Water Environ 30:2–21
Prach K, Hobbs RJ (2008) Spontaneous succession versus technical reclamation in the restoration of disturbed sites. Restor Ecol 16:363–366
Rufaut CG, Craw D, Law S, Druzbicka J (2018) Conservation of saline patches in central Otago needs better recognition of physical processes to secure future habitats. N Z J Bot 56:115–126
Smith LJ, Blowes DW, Jambor JL, Sego DC, Neuner M (2013) The Diavik waste rock project: particle size distribution and sulfur characteristics of low-sulfide waste rock. Appl Geochem 36:210–221
Sobolewski A, Sobolewski N (2022) Holistic design of wetlands for mine water treatment and biodiversity: a case study. Mine Water Environ 41:292–299
Sonter LJ, Ali SH, Watson JEM (2018) Mining and biodiversity: key issues and research needs in conservation science. Proc R Soc B 285:9
Sonter LJ, Dade MC, Watson JEM, Valenta RK (2020) Renewable energy production will exacerbate mining threats to biodiversity. Nat Commun 1:4174
Sposito G (2016) The chemistry of soils, 3rd edn. Oxford University Press, Oxford
Tang J, Bullard JW, Perry LN, Feng P, Liu J (2018) An empirical rate law for gypsum powder dissolution. Chem Geol 498:96–105
Tostevin R, Craw D, van Hale R, Vaughan M (2016) Sources of environmental sulfur in the groundwater system, southern New Zealand. Appl Geochem 70:1–16
Tropek R, Kadlec T, Karesova P, Spitzer L, Kocarek P, Malenovsky I, Banar P, Tuf IH, Hejda M, Konvicka M (2010) Spontaneous succession in limestone quarries as an effective restoration tool for endangered arthropods and plants. J Appl Ecol 47:139–147
Tropek R, Kadlec T, Hejda M, Kocarek P, Skuhrovec J, Malenovsky I, Vodka S, Spitzer L, Banar P, Konvicka M (2012) Technical reclamations are wasting the conservation potential of post-mining sites. a case study of black coal spoil dumps. Ecol Eng 43:13–18
Tropek R, Hejda M, Kadlec T, Spitzer L (2013) Local and landscape factors affecting communities of plants and diurnal Lepidoptera in black coal spoil heaps: implications for restoration management. Ecol Eng 57:252–260
Valente T, Gomes P, Pamplona J, Luisa de la Torre M (2012) Natural stabilization of mine waste dumps: evolution of the vegetation cover in distinctive geochemical and mineralogical environments. J Geochem Explor 123:152–161
Weightman E, Craw D, Rufaut C, Kerr G, Scott J (2020) Chemical evolution and evaporation of shallow groundwaters discharging from a gold mine, southern New Zealand. Appl Geochem 122:104766
Williams GJ (1974) Economic Geology of New Zealand. Austr Inst Min Metall Monogr 4
Wong MH (2003) Ecological restoration of mine degraded soils with emphasis on metal contaminated soils. Chemosphere 50:775–780
Acknowledgements
Funding for this research was provided by the University of Otago and Otago Regional Council. We appreciate the enthusiasm of Scott Jarvie (Otago Regional Council) and Ellery Mayhence, Clement Lagrue, Sasha Roselli, and Ross Curtis (Department of Conservation) who provided useful discussions and accompanied us on site visits to reserves. Mark Hesson provided helpful information on historic placer mining in the area. Roger Browne kindly allowed access to his land for part of this study. SEM observations were made with assistance of Gemma Kerr at Otago Micro and Nanoscale Imaging (OMNI), University of Otago. Constructive review comments from two journal referees, and editorial comments from Ann Maest, who improved the presentation of the ms.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Craw, D., Rufaut, C. & Pillai, D. Evolution of Alkaline Mine Drainage and Unique Biodiversity on Soil-Free Mine Substrates, Southern New Zealand. Mine Water Environ 42, 3–23 (2023). https://doi.org/10.1007/s10230-023-00913-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-023-00913-x