Abstract
The quality of mine drainage from sulphide-containing waste rock dumps is controlled by several factors. To characterize the effects of grain size on acid/neutral rock drainage production, kinetic tests were performed on samples from the Recsk porphyry skarn Cu–Zn deposit in Hungary, an area known to generate ARD. Five columns were used, each containing specific grain size ranges (1–2, 2–4, 4–8, 8–16, and 16–32 mm). Prior to the kinetic tests, a static test was performed for each grain size range to obtain total and available neutralizing potential (NP) and acid potential (AP). Total NP and AP values were roughly similar for all grain size ranges, while available NP increased as grain size decreased. The neutralization potential ratio for all grain size ranges was <1, which suggests a potentially acid-producing material. The kinetic tests, however, had contrasting results; a grain size of 1–4 mm produced a circumneutral pH, while grain size groups >4 mm produced pHs from 5.1 down to 3.6. Higher alkalinity values in the leach water were linked to the finer grain samples, primarily producing circumneutral pH. Grain size correlated with the sulphate release rate during the stable release period: the sulphate release rate was less at larger grain sizes. In contrast, sulphide oxidation calculated from oxygen consumption was highest for the intermediate grain size range, followed by the coarser and then the finer grains. The leaching of metals established an increasing concentration with increasing acidity without a very clear relationship to grain size. The established concept of increased metal mobility with decreasing pH applies, regardless of grain size.
Zusammenfassung
Die Qualität des Abflusses von sulfidhaltigen Halden wird durch einen Reihe von Faktoren bestimmt. Um den Einfluss der Korngröße auf die Bildung saurer/neutraler Sickerwässer zu bestimmen, wurden kinetische Tests durchgeführt mit Proben von der pyritischen Cu-Zn-Skarnerzlagerstätte Recsk in Ungarn, einem Gebiet, das für die Bildung sauer Sickerwässer bekannt ist. Fünf Säulen wurden benutzt, von denen jede Material eines bestimmten Korngrößenbereiches enthielt (1-2, 2-4, 4-8, 8-16 und 16-32 mm). Vor dem kinetischen Test wurde für jeden Korngrößenbereich ein statischer Test durchgeführt, um das gesamte und das verfügbare Neutralisationspotential (NP) und Versauerungspotential (VP) zu bestimmen. Die Gesamt-NP- und –AP-Werte waren für alle Korngrößenbereiche. Das verfügbare NP hingegen stieg mit kleiner werdender Korngröße an. Das Verhältnis NP/AP war für alle Korngrößen kleiner als eins. D.h., es handelte sich um potentiell Säure-produzierendes Material. Die kinetischen Tests hatten davon abweichende Ergebnisse. Bei Korngröße von 1 bis 4 mm war der pH-Wert etwa neutral während bei Korngrößen über 4 mm zu pH-Werten von 5,1 bis 3,6 führten. Höhere Alkalinität war an kleinere Korngrößen gebunden, die vorwiegend neutrale pH-Werte lieferten. Die Korngröße korrelierte mit der Freisetzungsrate von Sulfat während stabiler Freisetzungsperioden: Die Freisetzungsrate von Sulfat war kleiner bei großen Korngrößen. Im Gegensatz dazu war die Sulfidoxidation, die aus dem Sauerstoffverbrauch berechnet wurde, bei mittleren Korngrößen am höchsten, gefolgt von grßen und dann kleinen Korngrößen. Die Auswaschung von Metallen bewirkte höhere Konzentrationen mit steigender Azidität, aber ohne eine eindeutige Beziehung zur Korngröße. Das bekannte Konzept steigender Metallmobilität mit fallendem pH-Wert galt unabhängig von der Korngröße.
Resumen
La calidad del drenaje de mina producido por residuos de roca con sulfuros es controlada por varios factores. Para caracterizar los efectos del tamaño de grano sobre la producción de drenaje ácido/neutro (ARD), se realizaron ensayos cinéticos con muestras de un depósito de rocas porfiríticas skarn en Recsk, Hungría, que es un área reconocida como generadora de ARD. Se usaron cinco columnas, cada una conteniendo un rango específico de tamaño de grano (1-2, 2-4, 4-8, 8-16 y 16-32 mm). Antes de los ensayos cinéticos, se realizó un ensayo estático para cada rango de tamaño de grano para obtener las capacidades totales y disponibles de neutralización (NP) y el potencial de generación de ácido (AP). Los valores de NP total y AP fueron similares para todos los tamaños de grano mientras que NP disponible se incrementó a medida que el tamaño decrecía. La capacidad de neutralización para todos los rangos utilizados fue menor que 1, lo que sugiere que es un material potencialmente productor de ácido. Sin embargo, los ensayos cinéticos mostraron resultados contrastantes; un tamaño entre 1 y 4 mm produjo un pH cercano a la neutralidad mientras que para los rangos mayores a 4 mm se produjeron pHs desde 5,1 a 3,6. Los mayores valores de alcalinidad en el lixiviado fueron asociados a los menores tamaños de grano, produciendo pH casi neutro. El tamaño de grano correlacionó con la velocidad de sulfato liberada durante el período estable de liberación: esta velocidad fue menor a mayores tamaños de grano. En contraste, la oxidación de sulfuros calculada por el consumo de oxígeno fue máxima para el rango intermedio de tamaño de grano, seguida por el más grueso y finalmente por el más fino. La lixiviación de metales mostró un aumento en la concentración correlacionado con la acidez pero sin relación clara con el tamaño de grano. En conclusión, se observe un crecimiento de la movilidad de los metales a menores valores de pHs, independientemente del tamaño de grano.
抽象
含硫化物矸石堆产生的矿山废水水质受多种因素控制。为了分析粒度对矸石产酸与中和潜力的影响,选取Recsk(匈牙利)斑岩-矽卡型铜-锌矿石进行动态试验研究。五个淋溶柱内样品的粒度分别为1~2 mm、2~4 mm、4~8 mm、 8~16 mm和16~32 mm。在动态淋溶试验之前,首先通过每种粒度样品的静态试验测试它们的中和潜力 (NP)和产酸潜力(AP)。各粒度样品的中和潜力(NP)和产酸潜力(AP)大致相等,但中和潜力(NP)随粒度变小而增强;各粒度样品的中和潜力比都小于1,意味着所有样品都为潜在产酸矿石。各粒度样品的动态试验结果存在差异:粒径1~4 mm的样品溶滤水近中性,粒度大于4 mm样品的溶滤水pH值由5.1降至3.6,而细颗粒样品溶滤水的碱度增大而呈近中性。在稳定的离子溶滤释放阶段,硫酸盐的释放率与粒度相关,随颗粒增大而减小;相反,中等粒度样品的硫化物氧化率(依据氧气消耗量计算)最大,粗粒和细粒次之。金属离子的溶滤随酸度增大而增多,与样品粒度关系不明显,验证了金属离子活性随pH降低而增强的规律。








Similar content being viewed by others
References
Acero P, Cama P, Ayora C, Asta MP (2009) Chalcopyrite dissolution rate law from pH 1 to 3. Geol Acta 7(3):389–397
American Society of Testing Materials (ASTM) (2007) Standard test method for laboratory weathering of solid materials using humidity cell. American Soc for Testing and Materials. http://www.astm.org
Anderson M, Charer J, Nicholson R (1999) The oxygen consumption method (OCM): a new technique for quantifying sulphide oxidation rates in waste rock. In: Proceedings of the mining and the environment, pp 1133–1142
Bowell RJ, Sapsford DJ, Dey M, Williams KP (2006) Protocols affecting the reactivity of mine waste during laboratory-based kinetic tests. In: Proceedings of the 7th international conference on acid rock drainage (ICARD)
CEN/TC 292 (2009) Characterization of waste—static test for determination of acid potential and neutralisation potential of sulfidic waste. European Committee for Standardization
CEN/TR 16363 (2012) Characterization of waste—kinetic testing for assessing acid generation potential of sulphidic waste from extractive industries. European Committee for Standardization
Fergusson KD, Morin KA (1991) The prediction of acid rock drainage—lessons from the database. In: Proceedings of the 2nd international conference on the abatement of acidic drainage, vol 3, Montreal, QC, Canada, pp 85–106
Földessy J, Szebényi (2008) The mineralization of the Recsk Deeps and Lahoca—short geological overview. In: Proceedings of the University of Miskolc series A, mining, vol 73, pp 87–100. http://phd.lib.uni-miskolc.hu/JaDoX_Portlets/documents/document_13278_section_5414.pdf
Geidel G, Caruccio F, O’Hagan M (1983) An assessment of acid loads from various size fractions of sandstone and binder material. In: Proceedings of the symposium on surface mining, hydrology, sedimentology and reclamation, Lexington, KY, USA, pp 53–56
Hallberg KB (2010) New perspectives in acid mine drainage microbiology. Hydrometallurgy 104:448–453
Jambor JL, Blowes DW, Ritchie AIM (2003) Environmental aspects of mine wastes, vol 31. Mineralogical Association of Canada short course series, Vancouver
Lapakko K (1988) Prediction of acid mine drainage from Duluth Complex mining wastes in northeastern Minnesota. In: Proceedings of the mine drainage and surface mine reclamation conference, pp 180–190. http://www.asmr.us/Publications/Conference%20Proceedings/1988%20Vol%201/Lapakko%20180-190.pdf
Lapakko KA, Antonson DA (2006) Pyrite oxidation rates from humidity cell testing of greenstone rock. In: Proceedings of the 7th ICARD
Lapakko K, Olson M (2015) Scaling laboratory sulphate release rates to operational waste rock piles. In: Proceedings of the 10th ICARD
Lapakko K, Wessels J, Antonson D (1995) Long term dissolution testing of mine waste. US EPA Grant X-8200322-01-0, MN Department of Natural Resources. http://nepis.epa.gov/Adobe/PDF/2000EFQA.PDF
Lapakko K, Haub J, Antonson D (1998) Effects of dissolution time and particle size on kinetic test results. In: SME annual meeting and exhibition. http://wvmdtaskforce.com/proceedings/98/98LAP/98LAP.HTM
Lapakko KA, Engstrom JN, Antonson DA (2006) Effects of particle size on drainage quality from three lithologies. In: Proceedings of the 7th ICARD
Molnár F, Jung P, Kupi L, Pogány A, Vágó E, Viktorik O (2008) Epithermal zones of the porphyry–skarn–epithermal ore complexes at Recsk. In: Proceedings of the University of Miskolc series A, mining. http://www.matarka.hu/koz/ISSN_1219-008X/73k_2008_eng/ISSN_1219-008X_vol_73_2008_eng_099-128.pdf
Morin KA, Hutt NM (1994) An empirical technique for predicting the chemistry of water seeping from mine-rock piles. In: Proceedings of the international land reclamation and mine drainage conference and 3rd international conference on the abatement of acidic drainage, US Bureau of Mines SP 06A-94, pp 148–156
Morin KA, Hutt NM, Ferguson KD (1995). Measure rates of sulfide oxidation and acid neutralization in kinetic tests: statistical lessons from the database. In: Paper presented at Sudbury ‘95 conference on mining and the environment, pp 525–536. http://mdag.com/MDAG%20Paper%20Database/M0055%20-%20Morin%20et%20al%201995%20-%20Rates%20of%20Oxidation.PDF
Nordstrom DK (1982) Aqueous pyrite oxidation and the consequent formation of secondary iron minerals. In: Cedric KA, Fanning DS, Hossner IR (eds) Acid sulphate weathering. Soil Science of America special publication no. 10, pp 37–56
Parkhurst LB, Appelo CAJ (2012) User guide to PHREEQC (version 2). A computer program for speciation, batch reaction, one dimensional transport, and inverse geochemical calculation. USGS WRI Report 99-4259, Washington, DC
Parks GA (1990) Surface energy and adsorption at mineral–water interfaces: an introduction. Rev Mineral 23:133–175
Pearce S, Scott P, Weber P (2015) Waste rock dump geochemical evolution: matching lab data, models and predictions with reality. In: Proceedings of the 10th ICARD
Price WA (2009) Prediction manual for drainage chemistry from sulphidic geologic materials. MEND Report 1.20.1, Ottawa. http://www.abandoned-mines.org/pdfs/MENDPredictionManual-Jan05.pdf
Rimstidt DJ, Chermak JA, Gagen PM (1994) Chapter 1 rates of reaction of galena, sphalerite, chalcopyrite, and arsenopyrite with Fe(III) in acidic solutions. In: Alpers CN, Blowes DW (eds) Environmental geochemistry of sulfide oxidation. ACS symposium series. American Chemical Society, Washington, DC
Rosso KM, Becker U, Hochella M (1999) The interaction of pyrite 100 surfaces with O2 and H2O: fundamental oxidation mechanisms. Am Mineral 84:1549–1561
Rukezo G (2003) Drainage geochemistry of the Recsk-Lahoca mining area, Matra Mountains, Hungary. International Institute for Geo-Information Science and Earth Observation (ITC), Enschede
Schippers A, Breuker A, Blazejak A, Bosecker K, Kock D, Wright TL (2010) The biogeochemistry and microbiology of sulfidic mine waste and bioleaching dumps and heaps, and novel Fe(II)-oxidizing bacteria. Hydrometallurgy 104:342–350
Singer PC, Stumm W (1970) Acid mine drainage: the rate limiting step. Science 167:1121–1123
Smith L, LÓpez D, Beckie R, Morin K, Dawson R, Price W (1995) Hydrogeology of waste rock dumps. http://mend-nedem.org/wp-content/uploads/2013/01/1.Associate-Project-PA-1.pdf
Strömberg B, Banwart S (1999a) Experimental study of acidity-consuming processes in mining waste rock: some influences of mineralogy and particle size. Appl Geochem 14:1–16
Strömberg B, Banwart S (1999b) Weathering kinetics of waste rock from the Aitik copper mine, Sweden: scale dependent rate factors and pH controls in large column experiments. J Contam Hydrol 39:59–89
Walder I, Stormont J (2004) Thorough characterization of the hydrogeological and geochemical processes in waste rocks are essential for effective reclamation. In: Proceedings of the 6th international conference on clean technology for the mining industry
Acknowledgments
This research was made possible through funding and support from the Kjeøy Research and Education Center, Norway. This work was carried out in the Sustainable Resource Management Center of Excellence at the University of Miskolc as part of the TAMOP-4.2.2/A-11/1-KONV-2012-0049 “WELL aHEAD” project in the framework of the New Széchenyi Plan, funded by the European Union, co-financed by the European Social Fund. XRD, XRF, SEM–EDS, and all mineralogical characterization studies were performed at the University of Miskolc. We also acknowledge Dr. Ferenc Kristaly for conducting the XRD analysis and Ferenc Móricz for obtaining the data for the first 19 weeks of the experiment. We also extend our gratitude to Dr. Andrew Barnes of SRK-UK for his review and comments to help improve this manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
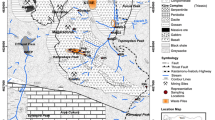
Fig. 1
(Supplemental) Location map of Recsk in Hungary (PDF 392 kb)
Fig. 2
(Supplemental) Kinetic column test set up (PDF 1621 kb)
Fig. 3
(Supplemental) XRD diffractograms for all of the grain size groups (TIFF 12556 kb)
Table 1
(Supplemental) Mineral liberation analysis (DOCX 12 kb)
Rights and permissions
About this article
Cite this article
Embile, R., Walder, I., Madai, F. et al. Grain Size Effects on Mine Water Quality and Acid/Neutral Rock Drainage Production in Kinetic Testing Using Recsk Porphyry Skarn Cu–Zn Deposit Rocks. Mine Water Environ 35, 421–434 (2016). https://doi.org/10.1007/s10230-015-0369-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10230-015-0369-x