Abstract
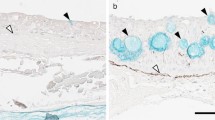
Wild anguillid eel larvae inhabit the ocean during their early life stages and never experience low-salinity water until the glass eel stage. The larvae show less mortality in half-diluted seawater than in full-strength seawater in captivity; however, physiological influences of environmental salinity on eel larvae have not been clarified. In this study, we compared the distributional and functional features of ionocytes between Japanese eel larvae acclimated to half-diluted and full-strength seawater. The mean tissue fluid osmolality in larvae acclimated to half-diluted seawater (300 mOsm/kg H2O) was slightly lower than in those (344 mOsm/kg H2O) acclimated to full-strength seawater. The density and opening size of ionocytes in the skin were not significantly different between the two salinities. Na+/K+-ATPase-immunoreactive ionocytes showed Na+/H+ exchanger-3 (NHE3) and cystic fibrosis transmembrane conductance regulator (CFTR) immunoreactions in their apical region and Na+/K+/2Cl- cotransporter-1 (NKCC1) immunoreaction in their basolateral region, suggesting that the skin ionocytes are involved in salt secretion in both salinities. In transmission electron microscopic observation, the ionocytes of larvae in full-strength seawater were characterized by the electron-dense cytoplasm, expanded tubular system and well-developed mitochondria, compared with those in half-diluted seawater, suggesting that the salt-secreting function was more activated in full-strength seawater than in half-diluted seawater. These results suggest that the energy metabolism cost of ion regulation could be lower in the intermediate salinity environment, which is closer to their osmolality than full-strength seawater. Hence, it is hypothesized that the saving of energy required for osmoregulation in half-diluted seawater could be favorable to better survival and growth of artificial eel larvae.




Similar content being viewed by others
References
Bœuf G, Payan P (2001) How should salinity influence fish growth? Comp Biochem Physiol C 130:411–423
Choe KP, Kato A, Hirose S, Plata C, Sindic A, Romero MF, Claiborne JB, Evans DH (2005) NHE3 in an ancestral vertebrate: primary sequence, distribution, localization, and function in gills. Am J Physiol Regul Integr Comp Physiol 289:R1520–1534
Cooper CA, Wilson JM, Wright PA (2013) Marine, freshwater and aerially acclimated mangrove rivulus (Kryptolebias marmoratus) use different strategies for cutaneous ammonia excretion. Am J Physiol Regul Integr Comp Physiol 304:R599–612
Dekker W, Casselman JM (2014) The 2003 Québec declaration of concern about eel declines—11 years later: are eels climbing back up the slippery slope? Fisheries 39:613–614
Dekker W, Casselman JM, Cairns DK, Tsukamoto K, Jellyman D, Lickers H (2003) Worldwide decline of eel resources necessitates immediate action: Québec declaration of concern. Fisheries 28:28–30
Désaunay Y, Guérault D (1997) Seasonal and long-term changes in biometrics of eel larvae: a possible relationship between recruitment variation and North Atlantic ecosystem productivity. J Fish Biol 51:317–339
Evans DH, Piermarini PM, Choe KP (2005) The multifunctional fish gill: dominant site of gas exchange, osmoregulation, acid–base regulation, and excretion of nitrogenous waste. Physiol Rev 85:97–177
Fridman S, Bron JE, Rana KJ (2012) Ontogenic changes in the osmoregulatory capacity of the Nile tilapia Oreochromis niloticus and implications for aquaculture. Aquaculture 356:243–249
Fukuda N, Miller MJ, Aoyama J, Shinoda A, Tsukamoto K (2013) Evaluation of the pigmentation stages and body proportions from the glass eel to yellow eel in Anguilla japonica. Fish Sci 79:425–438
Hiroi J, McCormick SD (2012) New insights into gill ionocyte and ion transporter function in euryhaline and diadromous fish. Respir Physiol Neurobiol 184:257–268
Hiroi J, McCormick SD, Ohtani-Kaneko R, Kaneko T (2005) Functional classification of mitochondrion-rich cells in euryhaline Mozambique tilapia (Oreochromis mossambicus) embryos, by means of triple immunofluorescence staining for Na+/K+-ATPase, Na+/K+/2Cl-cotransporter and CFTR anion channel. J Exp Biol 208:2023–2036
Hiroi J, Yasumasu S, McCormick SD, Hwang PP, Kaneko T (2008) Evidence for an apical Na-Cl cotransporter involved in ion uptake in a teleost fish. J Exp Biol 211:2584–2599
Imsland AK, Gúnnarsson S, Foss A, Stefansson SO (2003) Gill Na+, K+-ATPase activity, plasma chloride and osmolality in juvenile turbot (Scophthalmus maximus) reared at different temperatures and salinities. Aquaculture 218:671–683
Imsland AK, Gústarsson A., Gunnarsson S, Foss Árnason J, Arnarson I, Jónsson AF, Smáradóttir H, Thorarensen H (2008) Effects of reduced salinities on growth, feed conversion efficiency and blood physiology of juvenile Atlantic halibut (Hippoglossus hippoglossus L.). Aquaculture 274:254–259
Inokuchi M, Hiroi J, Watanabe S, Lee KM, Kaneko T (2008) Gene expression and morphological localization of NHE3, NCC and NKCC1a in branchial mitochondria-rich cells of Mozambique tilapia (Oreochromis mossambicus) acclimated to a wide range of salinities. Comp Biochem Physiol A 151:151–158
Kaneko T, Hasegawa S, Sasai S (2003) Chloride cells in Japanese eel (Anguilla japonica) during their early life stages and downstream migration. In: Aida K, Tsukamoto K, Yamauchi K (eds) Eel biology. Springer, Tokyo, pp 457–468
Kaneko T, Watanabe S, Lee KM (2008) Functional morphology of mitochondrion-rich cells in euryhaline and stenohaline teleosts. Aqua Biosci Monogr 1:1–62
Katoh F, Kaneko T (2003) Short-term transformation and long-term replacement of branchial chloride cells in killifish transferred from seawater to freshwater, revealed by morphofunctional observations and a newly established ‘time differential double fluorescent staining’ technique. J Exp Biol 206:4113–4123
Kuroki M, Fukuda N, Yamada Y, Okamura A, Tsukamoto K (2010) Morphological changes and otolith growth during metamorphosis of Japanese eel leptocephali in captivity. Coastal Mar Sci 34:31–38
Kuroki M, David R, Walker AM (2014) The importance of Anguillids: a cultural and historical perspective introducing papers from the World Fisheries Congress. Ecol Freshw Fish 23:2–6
Laiz-Carrión R, Sangiao-Alvarellos S, Guzmán JM, Martín del Río M, Soengas JL, Mancera JM (2005) Growth performance of gilthead sea bream Sparus aurata in different osmotic conditions: Implications for osmoregulation and energy metabolism. Aquaculture 250:849–861
Lambert Y, Dutil, J-D, Munro J (1994) Effects of intermediate and low salinity conditions on growth rate and food conversion of Atlantic cod (Gadus morhua). Can J Fish Aquat Sci 51:1569–1576
Lee KM, Kaneko T, Katoh F, Aida K (2006) Prolactin gene expression and gill chloride cell activity in fugu Takifugu rubripes exposed to a hypoosmotic environment. Gen Comp Endocrinol 149:285–293
Lee KM, Yamada Y, Okamura A, Tsukamoto K, Kaneko T (2013) Hyposmoregulatory ability and ion- and water-regulatory mechanisms during the leptocephalus stages of Japanese eel Anguilla japonica. Fish Sci 79:77–86
Leonard JB, Summers RG (1976) The ultrastructure of the integument of the American eel, Anguilla rostrata. Cell Tiss Res 171:1–30
Liu ST, Tsung L, Horng JL, Lin LY (2013) Proton-facilitated ammonia excretion by ionocytes of medaka (Oryzias latipes) acclimated to seawater. Am J Physiol Regul Integr Comp Physiol 305:R242–251
Miller MJ, Chikaraishi Y, Ogawa NO, Yamada Y, Tsukamoto K, Ohkouchi N (2012) A low trophic position of Japanese eel larvae indicates feeding on marine snow. Biol Lett 9:20120826–20120826
Mochioka N, Iwamizu M (1996) Diet of anguillid larvae: leptocephali feed selectively on larvacean houses and fecal pellets. Mar Biol 125:447–452
Mochioka N, Iwamizu M, Kanda T (1993) Leptocephalus eel larvae will feed in aquaria. Environ Biol Fish 36:381–384
Morgan JD, Iwama GK (1991) Effects of salinity on growth, metabolism, and ion regulation in juvenile rainbow and steelhead trout (Oncorhynchus mykiss) and fall chinook salmon (Oncorhynchus tshawytscha). Can J Aquat Sci 48:2083–2094
Morgan JD, Iwama GK (1999) Energy cost of NaCl transport in isolated gills of cutthroat trout. Am J Physiol 277:R631–R639
Ogasawara T, Hirano T (1984) Changes in osmotic water permeability of the eel gills during seawater and freshwater adaptation. J Comp Physiol B 154:3–11
Okamura A, Yamada Y, Mikawa N, Horie N, Utoh T, Kaneko T, Tanaka S, Tsukamoto K (2009a) Growth and survival of eel leptocephali (Anguilla japonica) in low salinity water. Aquaculture 296:367–372
Okamura A, Yamada Y, Horita T, Horie N, Mikawa N, Utoh T, Tanaka S, Tsukamoto K (2009b) Rearing eel leptocephali (Anguilla japonica Temminck & Schlegel) in a planktonkreisel. Aquacult Res 40:509–512
Okamura A, Horie N, Mikawa N, Yamada Y, Tsukamoto K (2014) Recent advances in artificial production of glass eels for conservation of anguillid eel populations. Ecol Freshw Fish 23:95–110
Otake T, Nogami K, Maruyama K (1993) Dissolved and particulate organic matter as possible food sources for eel leptocephali. Mar Ecol Prog Ser 92:27–34
Sasai S, Kaneko T, Hasegawa S, Tsukamoto K (1998) Morphological alteration in two types of gill chloride cells in Japanese eel (Anguilla japonica) during catadromous migration. Can J Zool 76:1480–1487
Sasai S, Katoh F, Kaneko T, Tsukamoto K (2007) Ontogenic change of gill chloride cells in leptocephalus and glass eel stages of the Japanese eel, Anguilla japonica. Mar Biol 150:487–496
Seo MY, Lee KM, Kaneko T (2009) Morphological changes in gill mitochondria-rich cells in cultured Japanese eel Anguilla japonica acclimated to a wide range of environmental salinity. Fish Sci 75:1147–1156
Seo MY, Mekuchi M, Teranishi K, Kaneko T (2013) Expression of ion transporters in gill mitochondrion-rich cells in Japanese eel acclimated to a wide range of environmental salinity. Comp Biochem Physiol A 166:323–332
Seo MY, Kuroki M, Okamura A, Tsukamoto K, Watanabe S, Kaneko T (2015) Occurrence of larval and adult types of ion-secreting ionocytes in Japanese eel Anguilla japonica. Ichthyol Res 62:487–494
Shinoda A, Aoyama J, Miller MJ, Otake T, Mochioka N, Watanabe S, Minegishi Y, Kuroki M, Yoshinaga T, Yokouchi K, Fukuda N, Sudo R, Hagihara S, Zenimoto K, Suzuki Y, Oya M, Inagaki T, Kimura S, Fukui A, Lee TW, Tsukamoto K (2011) Evaluation of the larval distribution and migration of the Japanese eel in the western North Pacific. Rev Fish Biol Fisheries 21:591–611
Shiraishi K, Kaneko T, Hasegawa S, Hirano T (1997) Development of multicellular complexes of chloride cells in the yolk-sac membrane of tilapia (Oreochromis mossambicus) embryos and larvae in seawater. Cell Tissue Res 288:583–590
Silva P, Solomon R, Spokes K, Epstein FH (1977) Ouabain inhibition of gill Na-K-ATPase: relationship to active chloride transport. J Exp Zool 199:419–426
Tanaka H (2015) Progression in artificial seedling production of Japanese eel Anguilla japonica. Fish Sci 81:11–19
Tsukamoto K, Umezawa A (1990) Early life history and oceanic migration of the eel, Anguilla japonica. La mer 28:188–198
Uchida K, Kaneko T, Miyazaki H, Hasegawa S, Hirano T (2000) Excellent salinity tolerance of Mozambique tilapia (Oreochromis mossambicus): elevated chloride cell activity in the branchial and opercular epithelia of the fish adapted to concentrated seawater. Zool Sci 17:149–160
Varsamos S, Diaz JP, Charmantier G, Blasco C, Connes R, Flik G (2002) Location and morphology of chloride cells during the post-embryonic development of European sea bass, Dicentrarchus labrax. Anat Embryol 205:203–213
Watanabe S, Niida M, Maruyama T, Kaneko T (2008) Na+/H+ exchanger isoform 3 expressed in apical membrane of gill mitochondrion-rich cells in Mozambique tilapia Oreochromis mossambicus. Fish Sci 74:813–821
Wilson JM, Reis-Santos P, Fonseca AV, Antunes JC, Bouca PD, Coumbra J (2007) Seasonal changes in ionoregulatory variables of the glass eel Anguilla anguilla following estuarine entry: comparison with resident elvers. J Fish Biol 70:1239–1253
Yanagie R, Lee KM, Watanabe S, Kaneko T (2009) Ontogenic change in tissue osmolality and developmental sequence of mitochondria-rich cells in Mozambique tilapia developing in freshwater. Comp Biochem Physiol A 154:263–269
Zadunaisky JA (1984) The chloride cell: The active transport of chloride and the paracellular pathways. In: Hoar WS, Randall DJ (eds) Fish Physiology, vol 10B. Academic Press, Orlando, pp 129–176
Acknowledgements
We thank Dr. Y. Yamada, Dr. N. Horie and Dr. N. Mikawa of the IRAGO Institute for their kind support during the experiment. This work was supported in part by a KAKEN C grant (No. 25450270) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Kuroki, M., Seo, M.Y., Okamura, A. et al. Morphofunctional features of ionocytes in Japanese eel Anguilla japonica leptocephali acclimated to half-diluted and full-strength seawater. Ichthyol Res 63, 487–495 (2016). https://doi.org/10.1007/s10228-016-0520-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10228-016-0520-0