Abstract
Background
Multiple sclerosis (MS) imposes high economic costs on society, but the patients and their families have to bear some of these costs.
Objective
We aimed to estimate the magnitude of these economic costs in Norway.
Method
We collected data through a postal questionnaire survey targeting 922 MS patients in Hordaland County, western Norway, in 2013–2014; 546 agreed to participate and were included. The questionnaire included clinical and demographic characteristics, volume and cost of MS-related resource use, work participation, income, government financial support, and disability status.
Results
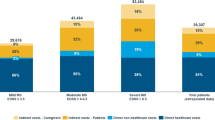
The mean annual total economic costs for the patients and their families were €11,603. Indirect costs accounted for 66% and were lower for women than for men. The direct costs were nearly identical for men and women. The costs increased up to Expanded Disability Status Scale score 6 except for steps between 3 and 4 where it remained nearly constant. The costs reduced from EDSS 6 to 8, and increased from 8 to 9. Lifetime costs ranged from €24,897 to €70,021 for patients with late disease onset and slow progression, and between €441,934 and €574,860 for patients with early onset and rapid progression.
Conclusion
The economic costs of MS impose a heavy burden on the patients and their families. Supplementing the information on the cost of MS to society, our finding should be included as background information in decisions on reimbursing and allocating public resources for the well-being of MS patients and their families.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system characterized by demyelination and axonal loss, leading to the accumulation of neurological functional impairment and disability. The progressive loss of functioning may result in a high level of disability, and several studies of the costs of MS have shown that it imposes high economic costs on society [1,2,3,4]. The patients and their families have to bear some of the costs, but data on their magnitude are limited.
A first partial estimate of the costs of MS to the patients and their families appeared in 1984 in an article on optimal financial compensation for people with chronic, disabling diseases [5]. Only three studies have comprehensively measured the total economic costs to the patients and their families. A study [6] estimated the total annual economic costs to society per patient in the United Kingdom in 1994 at three levels of disability. The study specified the part of the costs borne by the patients and their families, GBP 6098, adjusted for taxes and government financial support. A study in Canada [7] estimated the total annual and lifetime economic costs to society per patient, related to three levels of disability in 1995. Lifetime costs were estimated assuming the number of years at each disability level for an average patient. The costs to the patients and their families, CAD 19,850 annually and CAD 362,738 lifetime, were specified but not adjusted for government financial support. A study in the United States [8] estimated the total annual and lifetime economic costs to society per patient in 1994. The total lifetime economic costs were estimated assuming constant direct economic costs over time and differing indirect costs in four age intervals. The costs to the patients and their families, USD 24,121 annually and USD 1,575,200 lifetime, were specified. Later, studies on the cost of MS to the patients and their families have concentrated on direct [9,10,11] or indirect [12,13,14] costs. None of the above studies, except for the United Kingdom study from 1994, included income tax effects.
Research [15,16,17,18,19,20] has shown that economic pressure caused by MS has a wide range of serious negative effects on the well-being and quality of life of people with MS. Negative changes in their role definition include cessation of work, loss of self-esteem, feeling of letting their partner and children down or being a heavy burden on their partner, loss of social contact, and depression. In general, all family members are affected through loss of hopes and dreams. Although residents of Norway are universally covered by the welfare system and thus have equal access to health care services, the patients and their families have to bear some of the costs. To our knowledge, no study has yet been conducted combining annual and lifetime costs for MS patients and their families including direct economic costs and indirect economic costs adjusted for private and public disability pension, taxes and government financial support, in relation to disability status, and the speed of disease progression.
Objective
We aimed to study the costs of MS from the perspective of the patients and their families in Norway, defined as the reduction in family disposable income for the general consumption of goods and services caused by MS. We examined the annual total (direct plus indirect) economic costs related to disability status and lifetime costs related to the speed of disease progression.
Methods
Definitions
We defined direct economic costs as the out-of-pocket costs for MS-related resource use. We defined indirect economic costs as the net financial loss of the patients and their families resulting from reduced work participation caused by MS, minus government financial support.
Terms
Previous studies of the costs of MS have often used the Expanded Disability Status Scale (EDSS) [21] to express MS severity in addition to disability status. Our study needed a more precise notation. In this article, EDSS measures disability status. The Multiple Sclerosis Severity Score (MSSS) measures relative disease severity and the Global MSSS table disease progression [22].
We collected our information through a postal questionnaire survey sent to all MS patients (n = 922) in Hordaland County with a known address in August 2013, who had received a definite MS diagnosis according to the Poser [23] or McDonald [24] criteria. Two postal reminders were sent out in January and March 2014.
Questions were on demographic and clinical characteristics, and direct and indirect economic costs. Demographic and clinical questions covered sex, age at time of survey, age at disease onset and at first symptom, employment, level of education, and self-assessed disability status. General information on education, wages, and taxes was available from Statistics Norway and Norway’s tax authorities.
Self-assessed disability status was measured by 11 written health status descriptions that could be allocated to 10 full-points [1] on the EDSS. The patients indicated the description that described their own health status best. EDSS is the most commonly used disability status scale in MS research and includes eight functioning systems: pyramidal function, cerebellar function, brainstem function, sensory function, bladder and bowel function, visual function, and mental function. The original EDSS scale contains 11 full-point and 9 half-point levels of increasing disability [0 (no symptoms or signs), 1.0, 1.5, 2.0, 2.5–9.0, 9.5, and 10 (meaning dead)]. In our study, all half-point levels were collapsed with the full-point level below. Full-point level 10 (death) was excluded. The disability description in the collapsed list of disability levels that the patients indicated described their disability status best was used as the patient’s self-assessed EDSS level.
Questions on direct economic costs included volumes and costs to the patients and their families of MS-related resource use. The questions on volumes were on pre-defined resources, similar to a questionnaire validated in earlier studies of the costs of MS to European societies [25, 26]. All health resources routinely used by MS patients were included. Also, “Other” questions were added to each health resource category. We added questions on the cost of the use to the patients and their families to the questions on the volumes, as in previous analyses [1, 10]. To get a complete picture of the consumption of health resources caused by MS, the patients were instructed to include any MS-related health resource use, and also resource use for which the patients and their families did not directly bear the cost. Finally, we added questions on MS-related costs to clothing, nutrition, and traveling or vacation. Table 1 lists the health resource use covered by the questionnaire. Figure 1 shows an excerpt from the questionnaire. The patients were instructed to include all costs resulting from the use of health resources, such as transport and other related costs. The Norwegian Health Economics Administration regulates payment for the use of health resources. The use of approved health resources related to chronic diseases is free of charge after the out-of-pocket payments for such health resources exceed a threshold of about €250 each year. For the transport and other related costs, special rules apply. These costs are not automatically reimbursed. The patients must actually apply for reimbursement of the costs. The process is circumstantial. There are also limitations in the regulation of reimbursement of such costs. Costs are reimbursed for less than 30% of the transports. In practice, therefore, patients most often cover transport and other related costs irrespective of whether the €250 ceiling has been reached for the use of approved health resources. Therefore, the annual direct medical costs to the patients and their families may exceed the €250 ceiling. If patients reported purchasing or being provided with such resources, but did not provide a corresponding cost, we assumed that the threshold for the out-of-pocket payments for the use of health resources had been exceeded and that there had been no transport or other related costs, and assumed zero payment. This may have underestimated these costs. In the sensitivity analysis, we estimated the effect on total economic costs of inputing the mean costs for the patients using the resource instead of zero. MS patients may also have MS-related costs for medication, nutrition, household services, clothing, traveling, stays in rehabilitation institutions, equipment and mobility adaptations of a dwelling, and a car that are not funded or only partly funded by the Norwegian Health Economics Administration. If patients reported purchasing or being provided with such resources, but no actual outlays, the average filled-in amounts for the patients using the resource were imputed.
The recall period for MS-related resource use and costs was 1 month. An exception was a 1-year recall period for questions on equipment and mobility adaptations that are typically purchased by or provided to MS patients only once or a few times during the course of disease (adapting a dwelling or a car, ramps, lifts, wheelchairs, walkers, canes, crutches, and other helping aids).
The questions on indirect economic costs were on the patient’s income, disability pension percentage, government financial support, and reduced work hours for family members related to the MS. The recall period was 1 month except for a 1-year recall period for income.
We aimed to estimate the cost of MS to the patients and their families, as opposed to the cost of illness to MS patients and their families. Therefore, the respondents were informed to include only resource use and reduced work hours caused by their MS, as in previous cost of MS studies [25, 26].
No data were censored. The first author (BS) reviewed all returned questionnaires. All reported volume and cost figures were considered to be within realistic and not pre-defined limits. Thirteen patients did not return the questionnaire, but e-mailed or telephoned that their MS caused no symptoms or practical limitations. This corresponds to EDSS 0 in the questions on self-assessed EDSS in the questionnaire: “I have no problems and no limitations”. Their EDSS level was, therefore, set at 0. In accordance with the description, these patients should not be expected to answer yes to the questions if they had used any of the resources listed in Table 1. Their MS-related use of health resources and associated costs were set at zero. Similarly, these patients should not be expected to report any MS-related reduction in work participation, and their occupational status was set at “fully employed”.
Four patients were institutionalized and unable to complete the questionnaire. Their EDSS score was set at 9, and their only MS-related direct cost was set to payment for the institution. Institutionalized people in Norway do not pay explicitly for food, drugs, and necessary health services. For stays shorter than 60 days, they are charged a fixed daily rate. For stays exceeding 60 days, they are charged a part of their annual after-tax income, typically about €14,458 in 2013 (information from the Department of Health and Care, Municipality of Bergen, Hordaland County). This amount, reduced by €3614 for meals free of charge, was entered as the only cost of their resource use related to MS. In addition, an estimated after-tax average loss of income of €14,796 (information from Statistics Norway and Norway’s tax authorities) was added for institutionalized MS patients younger than the formal retirement age of 67 years in Norway.
We entered the reviewed and corrected information into a model programmed in Excel. The model calculated the annual direct economic costs for each respondent and their family by summing up the direct cost items, the mean costs for the patients using the resource in the recall period, and the mean annual cost for all respondents taking into account the proportion of patients having used the resource in the recall period. The costs that had been reported for a 1-month recall period were annualized by multiplying by 12.
Multiplying the reported cost of 1 month’s use by 12 may not reflect the annual costs of use for individuals. For the responding group, 1 month’s resource use and costs multiplied by 12 will approximate the costs.
The model calculated the indirect economic costs as each patient’s disability pension percentage multiplied by the difference between the mean net income for employed men and women in Hordaland County with the same distribution by education levels as the respondents, and the mean reported net income for the men and women in our study with a reported disability pension percentage of 100%. We subtracted government financial support. Finally, the model calculated the indirect economic costs of a family member’s reduced work hours because of the MS as the fraction of reduced work hours, multiplied by the mean net income for Hordaland County residents. The cost estimates reflect the price level during the period from September 1, 2013, to April 30, 2014, when the questionnaires were completed. Prices increased by 1.3% in Norway during this period.
Comparison of means of unrelated samples and linear regression analysis were used to estimate the potential effect on total economic costs of differences in age and gender proportions between the responders and non-responders.
Costs during the first 30 years after disease onset
To estimate the MS-related economic costs to the patients and their families during the first 30 years after disease onset, we combined our annual cost estimates with information on progression in the Global MSSS table. The MSSS measures relative disease severity by comparing individual EDSS scores with the distribution of EDSS in patients who have had MS for the same duration of time. The MSSS scores range from 0.00 to 9.99 and can be split into ten deciles, from 0.00–0.99 to 9.00–9.99 of increasing relative disease severity. The Global MSSS table shows the MSSS scores of patients on each EDSS level each of the first 30 years after disease onset in 9892 MS patients in Europe and Australia, and how the MSSS deciles progressed to higher EDSS scores during the period. The Global MSSS table shows the progression of the groups of patients each year with MSSS score in each decile of relative disease severity, but basically not the progression of individual patients. Individual patients’ MSSS scores may shift between different MSSS deciles during the period, but the stability of a person’s MSSS score over time was also shown in the European study, with mean and 80% range for changes over 5, 10, and 15 years. The mean change in MSSS was about zero. The 80% range for change in the MSSS was about ± 2.0, considerably less than ± 5.5, which would be expected if scores were taken at random, reflecting the moderate-to-strong correlation between MSSS measurements made at different time points on the same patient [22, 27].
We delineated our analysis of the 30-year costs to the patients and their families to (possibly hypothetical) patients with MSSS scores in the same decile of relative disease severity throughout the period. For these patients, ten cost intervals, one interval for patients with MSSS score in the same of the ten deciles of relative disease severity, can be estimated. Patients with higher MSSS scores reach higher EDSS scores earlier than patients with lower MSSS scores, i.e., they progress more rapidly, revealing increasing speed of progression with increasing MSSS scores.
We estimated 30-year costs for patients with disease onset at 32 years of age (the mean age at disease onset for the respondents) by substituting their EDSS levels each year according to the Global MSSS with the estimated annual total economic costs for the EDSS score. Due to the algorithm used to calculate MSSS scores, scores are missing for patients with MSSS score in some deciles of relative disease severity some years. Where MSSS scores were missing for the first year after disease onset, we conservatively assumed the EDSS score for the MSSS score below for our cost calculations. Where MSSS scores were missing for later years, we used the EDSS score for that MSSS score the preceding year.
We prepared “optimistic” and “pessimistic” estimates. Patients with MSSS score in a given decile of relative disease severity may be distributed on more than one EDSS score each year. In these cases, we used the lowest EDSS score for calculating the “optimistic” cost estimate (from the perspective of the patients and their families) and the highest score for the “pessimistic” estimate. In the Global MSSS table, patients with MSSS scores between 0.00 and 0.99, for example, 20 years after disease onset, are distributed on EDSS 0, 1.0, 1.5, and 2.0 (EDSS levels 0, 1, and 2 in our collapsed list of disability levels). We used the estimated cost for patients at EDSS 0 for our optimistic estimate and the cost for patients at EDSS 2 for the pessimistic estimate.
The amounts were discounted to account for differential timing of costs and summed up.
Lifetime cost
Thirty years is the period after disease onset for which empirical information on disease progression exists. The duration of the disease for individual patients varies with age at disease onset and may be longer than 30 years. The sensitivity analysis estimated the lifetime costs for patients with disease onset at 21 and 43 years of age (one SD in each direction from the mean age at disease onset). The cost estimates for years further than 30 years from disease onset are based on four assumptions: no further progression later than 30 years from disease onset, retirement age 67 years, no MS-related income loss after retirement, and life expectancy 75 years for MS patients.
In the literature, the recommended discount rates to account for the differential timing of costs vary [28, 29]. We used a real discount rate of 3% annually to the year of disease onset. The sensitivity analysis shows the effect on the total economic costs to the patients and their families of using nominal values and a 5% discount rate.
The Data Protection Officer of Haukeland University Hospital approved the study. We performed the statistical analysis using IBM SPSS Statistics version 22.
Results
Demographics
Of the 922 MS patients with a known address and a definite diagnosis in Hordaland County in August 2013, 583 responded. Thirty-seven patients refused to participate and 339 did not respond; 546 patients returned a completed questionnaire or provided required information by mail or phone, giving a response rate of 59%, of which 68% were women. The respondents’ mean age at time of survey was 53.2 years (± 12.9). The mean age at diagnosis was 38.3 years (± 11.4), and the age at the first symptom was 32.2 years (± 10.9). The median self-assessed EDSS score was 3.0 (IQR: 4), and the mean score was 3.4 (± 2.4). A total of 45% reported that they were fully (31%) or partly (14%) employed. Among the respondents, 12% had only primary or lower secondary school, 34% upper secondary school, and 43% had university education: 26% for up to 4 years and 17% for more than 4 years. Table 2 summarizes the clinical and demographic data.
The respondents had more women (not statistically significant) and a higher mean age (P = 0.003) than the non-respondents.
Annual direct economic costs
Direct medical costs
Direct medical costs include the costs of drugs, ambulatory care, and institutionalization.
The mean annual cost of drugs and complementary or alternative medication was €565. The cost of prescription drugs dominated at €297. Altogether 69% of the patients received prescription drugs during the 1-month recall period. Forty percent of the patients received disease-modifying treatment (Table 3). The Norwegian Health Economics Administration pays the full cost of disease-modifying treatment. The patients have transport costs for some treatments.
The mean annual cost for ambulatory care was €375. A total of 24% had received ambulatory care by a physician in hospital, mainly neurologists, in the 1-month recall period, and 23% by a general practitioner. Home visits by a nurse had been received by 5%, and 32% had visited a physiotherapist; 0.6% a psychologist; 1.1% a chiropractor; and 0.7% had visited an acupuncturist. The main cost of €78 was for physiotherapy visits, mainly because of frequent visits, on average 22 per year. In general, physiotherapy was free of charge for MS patients in 2013–2014 in Norway, but they often paid the transport costs. The cost of visits to physicians in hospital (€74) was close to the cost of visits to a physiotherapist. A total of 5% visited other professionals, mainly for facilitated physical training and “alternative” treatments.
The mean annual cost of institutionalization was €294. The proportion of patients reporting that they had been hospitalized in the 1-month recall period was 3%, and 7% reported outpatient hospital stays. Another 4% received rehabilitation and 2% stayed in a nursing home. The far highest annual cost was €170 for stays in a nursing home, where patients are charged about 80% of their net income after some deductions for stays longer than 60 days.
The mean annual direct medical costs amounted to €1234.
Direct non-medical costs
The direct non-medical costs include adaptations and equipment, assistance, and MS-related costs for clothing, nutrition, and traveling or vacation.
The mean annual cost of mobility adaptations, helping aids, and equipment was €1755 (Table 4). In the 1-year recall period, 5% of the patients had made mobility adaptations to their dwelling, 5% had purchased or been provided with an electric wheelchair, 4% with an electric scooter, and 6% with an adaptation of a car. The cost of mobility adaptations of a dwelling comprised the highest cost: €1482. Mobility adaptations of a dwelling are expensive, and the Norwegian Health Economics Administration does not generally pay for them.
The mean annual cost of assistance was €461: 4% had received services from a personal assistant in the 1-month recall period, 20% had purchased or been provided with household services, mainly house cleaning, and 6% other forms of assistance. Household services costs: €379.
The mean annual MS-related costs for clothing, nutrition, and traveling or vacation were €481. Costs in the 1-month recall period were reported by 11, 10, and 13% of the patients, respectively.
In total, the mean annual total direct non-medical costs were €2696, and thus the mean total annual direct economic costs were €3931 in 2013–2014: €3934 for women and €3924 for men (Table 5).
Annual indirect economic costs
We calculated annual indirect economic costs based on the patients’ MS-related net loss of income from work related to MS minus net public and private disability pension and government financial support, plus the net loss of income of family members related to the MS.
A total of 39% of the patients received 100% disability pension, and 14% reported various degrees of disability from 20 to 90% (Table 2), giving mean annual indirect costs of €6863 (Table 5). In addition, 24 patients (4%) reported that another family member had an average 40% reduced working hours because of the MS in the 1-month recall period, giving estimated mean annual indirect economic costs of €809. Reduced work participation due to sickness and rehabilitation absences causes no economic cost to Norwegian patients and their families as they receive full compensation during such absences.
The mean annual total indirect economic costs were €7672; €9989 for men and €6544 for women.
Total economic costs for MS patients and their families
When adding the annual direct (34%) and indirect (66%) economic costs, the mean annual total economic costs to the patients and their families were €11,603. Direct economic costs were nearly identical for women and men. The indirect costs were 34% lower for women, mainly because of lower salaries when disability pension was granted and because women received more government financial support.
The calculated direct economic costs were out-of-pocket costs, and we adjusted the indirect costs for tax effects, private and public disability pension, and government financial support. The calculated total MS-related economic costs, therefore, reduced the family disposable income for the general consumption of goods and services by its full amount.
The total economic cost increased by €147.2 per year of increasing age, starting at €3136. In the sensitivity analysis, we calculated the potential effect of the age difference between responders and non-responders on the average total economic costs to the patients and their families.
Economic costs related to EDSS score
Direct economic costs increased steadily up to full-point EDSS 6, declined from EDSS 6 to 8 before peaking at EDSS 9 (Table 6). The reduced costs of mobility adaptations of a dwelling were the largest item in the reduction from EDSS 6 to 8. Such adaptations are costly to the patients and their families and increasingly rare after patients have reached EDSS 6. The increase from EDSS 8 to 9 is explained by patients at EDSS 9 more often being institutionalized, where they are charged close to 80% of their net income for the stay.
Indirect economic costs basically increased up to full-point EDSS 7, except for a 9% decline between EDSS 3 and 4 caused by increased government financial support and by reduced income loss for family members. The costs were reduced by 31% from EDSS 7 to 8, mainly because of a higher percentage of retired patients at EDSS 8 (26%) than at EDSS 7 (23%). The increase from EDSS 8 to 9 is caused by reduced government financial support for patients at EDSS 9. A total of 67% of the patients at EDSS 9 were institutionalized. Institutionalized patients have limited access to government financial support.
The total economic costs increased up to full-point EDSS 6, except for between EDSS 3 and 4 where they remained nearly constant. The costs declined moderately from EDSS 6 to 7 and more markedly declined from EDSS 7 to 8. They then increased from EDSS 8 to 9.
Costs to patients and their families for the first 30 years after disease onset
For patients with disease onset at 32 years of age and MSSS score in the first decile of relative disease severity throughout the 30-year period covered by the Global MSSS table, the present value of the total estimated 30-year costs was between €27,832 and €85,546, discounted at 3% annually. For patients with MSSS score in the 10th decile throughout the period, the estimated costs were between €353,375 and €437,854. Table 7 gives cost intervals for patients with MSSS scores in all the ten deciles of relative disease severity throughout the 30-year period. Because of the decline in mean annual total economic costs between EDSS 6 and 8, the 30-year costs for patients with MSSS scores in the 7th to 10th decile of disease severity are within a relatively narrow interval.
We estimated the lifetime costs for patients with disease onset at 21 and 43 years of age. The estimated lifetime costs to patients with MSSS score in the first decile of relative disease severity throughout the course of disease and disease onset at 43 years of age are estimated between €24,897 and €70,021 and between €330,850 and €414,718 for patients with MSSS score in the 10th decile. For patients with disease onset at 21 years of age, the corresponding numbers are between €35,680 and €129,618 and between €441,934 and €574,860 (Table 8).
Sensitivity analysis
The effect on total economic costs of imputing the mean costs for the users of the resource instead of zero costs when respondents indicated using a resource, but did not state an amount is + 1.4%. (Table 8). The effect of adjusting for differences in age between respondents and non-respondents is − 1.3%. Finally, the effects on lifetime costs of using nominal values and of discounting at 5% are shown.
Discussion
We examined the annual total MS-related economic costs for the patients and their families during 2013–2014, as measured by the reduction in family disposable income for the general consumption of goods and services caused by MS. The mean annual total economic costs were €11,603 per patient, including €3931 in direct costs and €7672 in indirect costs. The direct costs were similar for men and women. The indirect costs were 34% lower for women. The estimated present value of the lifetime costs for patients with disease onset at 21 years of age was between €35,680 and €129,618 (discounted at 3%) and between €441,934 and €574,860 for patients with MSSS scores in the 1st and 10th decile of disease severity throughout the course of disease.
Of the three comprehensive studies in the mid-1990s on the total annual economic costs to the patients and their families, the studies in the United Kingdom and Canada [6, 7] related the costs to disability levels. The costs increased with three broadly defined levels of escalating disability in both studies. In our study, the costs increased up to full-point EDSS 6, except for between EDSS 3 and 4 where they remained nearly constant. They declined moderately from EDSS 6 to 7 and more markedly declined from EDSS 7 to 8, and increased from EDSS 8 to 9. If we collapse our disability status scores into three broader levels, comparable to those in the studies in the United Kingdom and Canada, our results also show steadily increasing costs with escalating disability.
We do not compare the results further. The welfare system in the United States, the United Kingdom, and Canada may have changed since 1994–1995. Further, differences between the estimated costs to the patients and their families based on updated cost amounts would have been influenced by the choice of indexes to account for inflation, exchange rates used for currency conversion, discount rate to account for the differential timing of costs, and the definition of “lifetime” in the studies. Differences might also have been caused by methodological issues such as differences in including and valuing cost items, the representativeness of the study population, and the methods for collecting and managing data. Adjusting correctly for all these factors is impossible. This highlights a need for more up-to-date and standardized studies of the costs of MS from the perspective of the patients and their families.
We defined the cost of MS to the patients and their families as the reduction in family disposable income for the general consumption of goods and services caused by MS. Some relevant costs will be obvious. For example, required part-payment for consultations, drugs, etc., or the total cost of treatments and purchases of nutrition products is not reimbursed by the Norwegian Health Economics Administration. Some costs will be far less obvious. For example, replacing cloths have been damaged by the wheel chair or spilled to while eating, and choosing a more expensive means of travel, apartment, or hotel to be able to function well during vacations, etc. We aimed to give a complete picture of all these costs. The patients were given this information in the fill-in guidance to the questionnaire.
Data for cost of illness analysis can be extracted from national registries and statistics or collected directly from a limited sample of people with illness, usually by questionnaires. Both methods have strengths and weaknesses. Data from national registries and statistics are generally objective, representative for the population studied and not influenced by subjective evaluation. However, national registries and statistics may not contain all the information needed for the study. The tools to collect data directly from people with illness can be tailored to provide all needed information. However, the data may be influenced by subjective evaluation and exposed to recall error and error caused by misunderstanding of questions. In Norway, national registry and statistical information on the out-of-pocket costs to the patients and their families for MS-related resource use are generally not available, and we did not have access to existing information on indirect costs at the level detail required for our study because of Norway’s data protection rules. We, therefore, collected our information through a postal questionnaire survey sent to MS patients in Hordaland County, Norway, but made efforts to reduce the potential effects of the weaknesses with this approach.
To reduce recall error, the questions on the volumes and costs of MS-related resource use and reduced work participation had mostly a 1-month recall period. The exception was a 1-year recall period for resources that are purchased by or provided to patients only once or a few times during the course of disease, i.e., adapting a dwelling or a car, ramps, lifts, wheelchairs, walkers, canes, crutches, and other helping aids.
For resources that are typically purchased by or provided to MS patients only once or a few times during the course of disease, longer recall periods than 1 year might have been an alternative. Whether this would have provided that more correct cost estimates is uncertain, however, and a 1-year recall period for using such resources has been typical in several studies of the cost of MS. Further, in Norway, the costs to the patients and their families of acquiring all except one of these resources, mobility adaptation of a dwelling, are low because of large subsidies. They are normally also acquired more than once during the course of disease, and choosing longer recall periods would probably have affected the total cost estimate marginally. A dwelling, however, is most typically mobility adapted only once, is expensive, and is mostly paid for by the patients. As a check on the reporting of this dominant direct cost item, the patients who reported adapting a dwelling were contacted by telephone to verify that they had understood and answered the question correctly. The answers revealed that in two cases, the adaptation could have been made over a longer period than the year before the questionnaire was completed. In these two cases, we divided the reported cost by two before being included.
To reduce misunderstanding of questions, the questions on the volumes and costs of health resources used and on reduced work participation were similar to the previous cost of MS studies except for questions on MS-related costs to clothing, nutrition, and traveling or vacation. These costs are well known to MS patients in Norway since government financial support can be grated to cover part of the costs.
To reduce subjective evaluations, all questions except for one were factual: the number of visits to physiotherapists, the out-of-pocket cost of the visits, etc. The exception was the self-assessed EDSS score. The questions on self-assessed EDSS had been tested on a limited sample of patients and were well correlated with neurologist-rated EDSS scores [1]. The patients were explicitly informed to include only resource use caused by their MS. This added an extra element of subjective evaluation. For studies of the costs of illness, distinguishing how much of the use of health resources and reduced work participation the disease being investigated causes from how much other health problems cause may be difficult. For MS, however, this is considered to be less of a problem, since the effects of MS are rather well defined [30], and studies on self-reporting data from MS patients have been shown to be highly accurate despite potential cognitive difficulties and general recall bias [26].
We delineated our study of the 30-year costs to the patients and their families to (possibly hypothetical) patients with MSSS scores in the same decile of relative disease severity throughout the period. This enabled us to illustrate the relation between costs and the speed of disease progression with the costs of ten schematic, but realistic progression paths of increasing speed of progression. Other progression paths are possible, but all will have cost estimates in the interval between our optimistic estimate for patients with MSSS scores in the first decile of relative disease severity, and the pessimistic estimate for the patients with MSSS scores in the 10th decile of relative disease severity. Our 30-year cost estimates, therefore, span the range of possible 30-year costs to the patients and their families. The data used to develop the Global MSSS reflect the situation in 2005 for patients with disease onset from 1 to 30 years earlier. The situation in 2005 may not be representative for the current situation. The increased use of gradually more efficacious disease-modifying therapies has especially slowed the disease progression. Thus, the deciles of relative disease severity in an updated Global MSSS have probably shifted somewhat to the left, and accordingly changed the cost for patients with MSSS scores in most of the deciles. The potential for reduced costs would have been greatest for patients with MSSS scores in the first six deciles. The potential cost reductions for patients with MSSS scores in the 7th to 10th deciles would have been more limited because of the relatively narrow interval of costs for patients with MSSS scores in these deciles. But no data are available today, however, on the possibly slowed progression.
In calculating the 30-year and lifetime costs, we incorporated the costs of adapting a dwelling in the yearly costs at each EDSS level. This may have led to overestimating these costs for patients who stay longer than average at EDSS levels with a high cost of adapting a dwelling, primarily patients at EDSS 6 and, to lesser extent, EDSS 5 and 7. Ideally, the costs of adapting a dwelling should have been incorporated in the cash flows using an incidence-based method. For today, however, so many simplifying assumptions would have to be made that the adjustments to the cash flows would seem unrealistic. The cost of adapting a dwelling in our study was the out-of-pocket costs for the adaptation. A hidden cost will be a reduced market value for the dwelling, since other people neither need nor fancy dwellings adapted for people with disabilities. We did not include this loss in market value in the costs.
The respondents were significantly younger than the non-respondents. The sensitivity analysis quantified the effect of this on the total cost estimate. The non-respondents may also have different MS-related resource use and costs than the respondents. We cannot correct our cost estimates for the potential effect of this. The response rate in our study (59%) is high for a questionnaire-based study of the cost of MS. In studies in nine European countries [1], only the one for Sweden had a higher response rate (75%) and the other ranged between 19 and 52%.
The Global MSSS has been validated for use in comparing the disease progression in groups of patients. Disease fluctuations preclude using it to predict the future disability of an individual. We used the information on disease progression in the Global MSSS table to estimate 30-year and lifetime costs to the patients and their families for ten groups of patients with MSSS scores in the same decile of relative disease severity throughout the course of disease.
The data were collected in Hordaland County. One limitation is that the cost effects of the disease in Hordaland County may not be representative of the cost effects in other parts of Norway. The Norwegian Multiple Sclerosis Competence Centre is located at the Department of Neurology of Haukeland University Hospital in Hordaland County, and numerous MS studies conducted at the Department may increase the contact frequency of the patients to the Department. However, epidemiological studies from Hordaland have shown similar prevalence and characteristics of the MS population, and these are comparable to other parts of the country [31]. In addition, national surveys of the use of disease-modifying therapies have shown that the average treatment frequency in Hordaland County has been similar to that of the country as a whole [32]. Hordaland County has about 10% of the MS patients in Norway and comprises both rural and urban areas like most other counties in Norway. This should contribute to making the MS population in Hordaland County reasonably representative for the national MS population.
Conclusion
It has long been recognized that MS imposes high economic costs on society. In Norway, all members of society share most of the costs. A part, however, has to be borne by the patients and their families themselves. Our study revealed that this imposes a considerable economic burden on the patients and their families, even in Norway’s welfare state with a well-developed social security system. Supplementing the information on the costs of MS to society that has more typically been provided by studies of the costs of MS, our finding should be included as background information in decisions on reimbursing and allocating resources for the well-being of the MS patients and their families.
References
Kobelt, G., Berg, J., Lindgren, P., Fredrikson, S., Jönsson, B.: Costs and quality of life of patients with multiple sclerosis in Europe. J. Neurol. Neurosurg. Psychiatry 77, 918–926 (2006)
Karampampa, K., Gustavsson, A., Miltenburger, C., Eckert, B.: Treatment experience, burden and unmet needs (TRIBUNE) in MS study: results from five European countries. Mult. Scler. 18(Suppl. 2), 7–15 (2012)
Adelman, G., Rane, S.G., Villa, K.F.: The cost burden of multiple sclerosis in the United States: a systematic review of the literature. Med. Econ. 16, 639–647 (2013)
Naci, H., Fleurence, R., Birt, J., Duhig, A.: Economic burden of multiple sclerosis: a systematic review of the literature. Pharmacoeconomics 28, 363–379 (2010)
Inman, R.P.: Disability indices, the economic cost of illness, and social insurance: the case of multiple sclerosis. Acta Neurol. Scand. Suppl. 101, 46–55 (1984)
Holmes, J., Madgwick, T., Bates, D.: The cost of multiple sclerosis. Br. J. Med. Econ. 8, 181–193 (1995)
Canadian Burden of Illness Study Group: Burden of illness of multiple sclerosis. I. Cost of illness. Can. J. Neurol. Sci. 25, 23–30 (1998)
Whetten-Golldstein, K., Sloan, F.A., Goldstein, L.B., Kulas, E.D.: A comprehensive assessment of the cost of multiple sclerosis in the United States. Mult. Scler. 4, 419–425 (1998)
Palmer, A.J., Colman, S., O’Leary, B., Taylor, B.V., Simmons, R.D.: The economic impact of multiple sclerosis in Australia in 2010. Mult. Scler. 19, 1640–1646 (2013)
Fogarty, E., Walsh, C., McGuigan, C., Tubridy, N., Barry, M.: Direct and indirect economic consequences of multiple sclerosis in Ireland. Appl. Health Econ. Health Policy 12, 635–645 (2014)
da Silva, N.L., Takemoto, M.L., Damasceno, A., Fragoso, Y.D., Finkelsztejn, A., Becker, J., Gonçalves, M.V., Tilbery, C., de Oliveira, E.M., Callegaro, D., Boulos, F.C.: Cost analysis of multiple sclerosis in Brazil: a cross-sectional multicenter study. BMC Health Serv. Res. 16, 102 (2016)
Jennum, P., Wanscher, B., Frederiksen, J., Kjellberg, J.: The socioeconomic consequences of multiple sclerosis: a controlled national study. Eur. Neuropsychopharmacol. 22, 36–43 (2012)
Pfleger, C.C.H., Flachs, E.M., Koch-Henriksen, N.: Social consequences of multiple sclerosis. I. Early pension and temporary unemployment—a historical perspective cohort study. Mult. Scler. 16, 121–126 (2010)
Wiberg, M., Friberg, E., Stenbeck, M., Alexanderson, K., Norlund, A., Hillert, J., Tinghøg, P.: Sources and level of income among individuals with multiple sclerosis compared to the general population: a nationwide population-based study. Mult. Scler. 21, 1–12 (2015)
Catanzaro, M., Weinert, C.: Economic status of families living with multiple sclerosis. Int. J. Rehab. Res. 15, 209–218 (1992)
Hakim, E.A., Bakheit, A.M.O., Bryant, T.N., Roberts, M.W.H., McIntosh-Michaelis, S.A., Spackman, A.J., Martin, J.P., McLellan, D.L.: The social impact of multiple sclerosis—a study of 305 patients and their relatives. Disabil. Rehabil. 22, 280–293 (2000)
McCabe, M.P., De Judicibus, M.: The effect of economic disadvantage on psychological well-being and quality of life among people with multiple sclerosis. J. Health Psychol. 10, 163–173 (2005)
De Juducibus, M.A., McCabe, M.P.: The impact of financial costs of multiple sclerosis on quality of life. Int. J. Behav. Med. 14, 3–11 (2007)
McCabe, M.P., O’Connor, E.J.: A longitudinal study of economic pressure among people living with a progressive neurological illness. Chronic Ill. 5, 177–183 (2009)
Green, G., Todd, J., Pevalin, D.: Biographical disruption associated with multiple sclerosis: using propensity scoring to assess the impact. Soc. Sci. Med. 65, 524–535 (2007)
Kurtzke, J.F.: Rating neurological impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology 33, 1444–1452 (1983)
Roxburgh, R.H., Seaman, S.R., Masterman, T., Hensiek, A.E., Sawcer, S.J., Vukusic, S., Achiti, I., Confavreux, C., Coustans, M., le Page, E., Edan, G., McDonnell, G.V., Hawkins, S., Trojano, M., Liguori, M., Cocco, E., Marrosu, M.G., Tesser, F., Leone, M.A., Weber, A., Zipp, F., Miterski, B., Epplen, J.T., Oturai, A., Sørensen, P.S., Celius, E.G., Lara, N.T., Montalban, X., Villoslada, P., Silva, A.M., Marta, M., Leite, I., Dubois, B., Rubio, J., Butzkueven, H., Kilpatrick, T., Mycko, M.P., Selmaj, K.W., Rio, M.E., Sá, M., Salemi, G., Savettieri, G., Hillert, J., Compston, D.A.: Multiple Sclerosis Severity Score: using disability and disease duration to rate disease severity. Neurology 64, 1144–1151 (2005)
Poser, C.M., Paty, D.W., Scheinberg, L.: New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann. Neurol. 13, 227–231 (1983)
Polman, C.H., Reingold, S.C., Banwell, B., Clanet, M., Cohen, J.A., Filippi, M., Fujihara, K., Havrdova, E., Hutchinson, M., Kappos, L., Lublin, F.D., Montalban, X., O’Connorm, P., Sandberg-Wollheim, M., Thompson, A.J., Waubant, E., Weinshenker, B., Wolinsky, J.S.: Diagnostic criteria for multiple sclerosis: 2010 revision to the McDonald criteria. Ann. Neurol. 69, 292–302 (2011)
Henriksson, F., Fredrikson, S., Masterman, T., Jönsson, T.: Costs, quality of life and disease severity in multiple sclerosis: a cross-sectional study in Sweden. Eur. J. Neurol. 8, 27–35 (2001)
Kobelt, G., Lindgren, P., Smala, A., Jönsson, B.: Group GCoMS: costs and quality of life in multiple sclerosis: a cross-sectional observational study in Germany. Eur. J. Health Econ. 2, 60–68 (2001)
Gray, O.M., Jolley, D., Zwannikken, C., Troyano, M., Grand´Maison, F., Duquette, P., Grammond, P., Bergamaschi, R., Giuliani, R., Taffi, R., Hupperts, R.M.M., Petersen, T., Boz, C., Rio, M.E., Van Pesch, V., Roulette, E., Lechner-Scott, J., Izquierdo. G., Cristiano, E., Pöhlau, D., Fiol, M., Oleschko Arruda, W., Deri, N., Flechter, S., Paine, M., Savino, A., Cabrera-Gomez, J.A., Hoffmann, M.W., Butzkueven, W.: The Multiple Sclerosis Severity Score (MSSS) re-examined: EDSS rank stability in the MSBase dataset increases 5 years after onset of multiple sclerosis. Neurol. Asia 13, 217–219 (2008)
NICE Decision Support Unit: Update to the NICE Technology Appraisals Methods Guide (2013)
Drummond, M.F., Sculper, M.J., Torrance, G.W., O’Brien, B.J., Stoddard, G.L.: Methods for the Economic Evaluation of Health Care Programs, 3rd edn, pp. 72–78 . Oxford University Press (2007)
Kobelt, G., Berg, J., Lindgren, P., Jönsson, B.: Costs and quality of life in multiple sclerosis in Europe; method of assessment and analysis. Eur. J. Health Econ. 7, 5–13 (2006)
Grytten, N., Glad, S,B,, Aarseth, J.H., Nyland, H., Midgaard, R., Myhr, K-M.: A 50-year follow-up of the incidence of multiple sclerosis in Hordaland County, Norway. Neurology 66, 182–186 (2006)
Torkildsen, O.E., Grytten, N., Myhr, K.-M.: Immunomodulatory treatment of multiple sclerosis in Norway. Acta Neurol. Scand. Suppl. 187, 46–50 (2007)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent and confidentiality
Patients gave written consent. Information was linked to patients via numerical codes. The codes were stored in a separate file at Haukeland University Hospital’s research database. The file with the codes was deleted January 15th 2016. The project was approved by the data protection officer at Haukeland University Hospital. No further approval was required.
Conflict of interest
None one of the authors have reported conflict of interest.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Svendsen, B., Grytten, N., Bø, L. et al. The economic impact of multiple sclerosis to the patients and their families in Norway. Eur J Health Econ 19, 1243–1257 (2018). https://doi.org/10.1007/s10198-018-0971-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-018-0971-5