Abstract
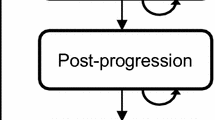
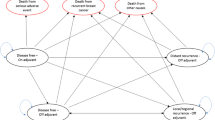
Five years with the aromatase inhibitors letrozole or anastrozole is clinically superior to 5 years tamoxifen in postmenopausal women with early breast cancer. This paper analyses the cost-effectiveness of the aromatase inhibitors compared to tamoxifen using the same health economic model. A Markov model describes lifetime incidence of breast cancer events and treatment-related adverse events. Probabilities of disease progression, adverse events, and utility values were estimated using secondary sources; costs of breast-cancer care were obtained from a primary costing study. The incremental cost per QALY gained of letrozole vs. tamoxifen is £10,379 (95% CI £6,705–23,574), and of anastrozole versus tamoxifen is £11,428 (95% CI £6,211–48,795). If a 5-year carry over effect for the reduction in breast cancer events is assumed, the incremental costs per QALY gained compared to tamoxifen are £6,253 (95% CI £3,675–14,766) for letrozole and £7,015 (95% CI £3,316–31,997) for anastrozole. Five years of letrozole or anastrozole therapy is cost-effective in postmenopausal women with early breast cancer. Though the respective confidence intervals show significant overlap, letrozole has a 95% probability of being more cost-effective than tamoxifen at a £20,000 QALY value, whilst anastrozole has an 85% probability.



Similar content being viewed by others
References
Harris, J.R., Morrow, M., Bonadonna, G., Cancer of the breast. In: De Vita et al. Cancer. Principles and Practice of Oncology, 4th edn. Lippencott, Philadelphia (1993)
Glick, J.H.: Adjuvant therapy for node-negative breast cancer. In: Fowble, B., Goodman, R.L., Glick, J.H., Rosato, E.F. (eds.) Breast Cancer Treatment. A Comprehensive Guide to Management. Mosby Year Book, St Louis, (1991)
Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 365, 1687–1717 (2005)
The Breast International Group (BIG) 1-98 Collaborative Group, A comparison of Letrozole and Tamoxifen in postmenopausal women with early breast cancer. New Eng. J. Med. 353, 2747–2757 (2005)
ATAC Trialists’ Group, Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer, Lancet 365, 60–62 (2005)
Women fighting for Herceptin, http://www.herceptin.dorothygriffiths-bcaf.org.uk/news.html (accessed 10 April 2007)
Karnon, J., Johnston, S.R.D., Delea, T., Barghout, V., Thomas, S., Papo, N.L.: Letrozole is cost-effective versus tamoxifen as adjuvant therapy in postmenopausal women with early breast cancer: BIG-1-98. Eur. J. Cancer. Suppl. 3(2), 96 (2005)
Delea, T.E., Karnon, J., Thomas, S.K., Barghout, V., Papo, N.L., Johnston, S.R.D.: Cost-effectiveness of letrozole versus tamoxifen as initial adjuvant therapy in hormone-receptor positive postmenopausal women with early breast cancer from a US perspective. 29th Annual San Antonia Breast Cancer Symposium, 14–17 December, (2006)
Hillner, B.E.: Benefit and projected cost-effectiveness of anastrozole versus tamoxifen as initial adjuvant therapy for patients with early-stage estrogen receptor-positive breast cancer. Cancer 101, 1311–1322 (2004)
Brown, R., Benedict, A., Mansel, R.: Cost-utility analysis of anastrozole versus tamoxifen as adjuvant therapy in postmenopausal women with early breast cancer: a UK national health service perspective, ISPOR 7th Annual European Congress, 24–26 October, Hamburg (2004)
Sonnenberg, F.A., Beck, J.R.: Markov models in medical decision making: a practical guide. Med. Decis. Mak. 13, 322–338 (1993)
National Institute for Clinical Excellence, Guide to the Methods of Technology Appraisal, April (2004)
Department of Health, Financial Matters, Appendix 1––Health Service Cost Index, May (2004)
Briggs, A.H.: A Bayesian approach to stochastic cost-effectiveness analysis. Health Econ. 8, 257–261 (1999)
Karnon, J., Brown, J.: Tamoxifen plus chemotherapy versus tamoxifen alone as adjuvant therapies for node positive postmenopausal women with early breast cancer. Pharmacoeconomics. 20(2), 119–137 (2002)
Moran, M.S., Haffty, B.G.: Local-regional breast cancer recurrence: prognostic groups based on patterns of failure. Breast J 8(2), 81–87 (2002)
Haylock, B.J., Coppin, C.M.L., Jackson, J. et al.: Locoregional first recurrence after mastectomy: prospective cohort studies with and without immediate chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 46(2), 355–362 (2000)
Schmoor, C., Sauerbrei, W., Bastert, G. et al.: Role of isolated locoregional recurrence of breast cancer: results of four prospective studies. J. Clin. Oncol. 18(8), 1696–1708 (2000)
Kamby, C., Sengelov, L.: Pattern of dissemination and survival following isolated locoregional recurrence of breast cancer. Breast Cancer Treat. Res. 45, 181–192 (1997)
Borner, M., Bacchi, M., Goldhirsch, A. et al.: First isolated locoregional recurrence following mastectomy for breast cancer: results of a phase III multicenter study comparing systemic treatment with observation after excision and radiation. J. Clin. Oncol. 12, 2071–2077 (1994)
Toonkel, L.M., Fix, I., Jacobsen, L.H. et al.: The significance of local recurrence of carcinoma of the breast. Int. J. Radiat. Oncol. Biol. Phys. 9, 33–39 (1983)
Mouridsen, H., Gershanovich, M., Sun, Y. et al.: Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a phase III study of the International Letrozole Breast Cancer Group. J. Clin. Oncol. 19(10), 2596–2606 (2001)
Actuary’s Department, UK Government. Interim life tables for England and Wales 1999–2001 http://www.gad.uk/Life_Tables/Historical_Interim_life_tables.htm
Ragaz, J., Coldman, A.: Survival impact of adjuvant tamoxifen on competing causes of mortality in breast cancer survivors, with analysis of mortality from contralateral breast cancer, cardiovascular events, endometrial cancer, and thromboembolic episodes. J. Clin. Oncol. 16, 2018–2024 (1998)
British National Formulary, British Medical Association and the Royal Pharmaceutical Society of Great Britain, March (2006)
NHS Executive. The new NHS 2004 reference costs. London: Department of Health, (2004)
Netten A, Curtis L, Unit Costs of Health and Health Care 2002, PSSRU, http://www.pssru.ac.uk/UC2002.htm
Kanis, J.A., Brazier, J.E., Stevenson, M. et al.: Treatment of established osteoporosis: a systematic review and cost–utility analysis. Health Technology Assessment 6(29), (2002)
Sorensen, S., Brown, R., Benedict, A., Flood, E., Revicki, D.: Patient-rated utilities in postmenopausal early breast cancer: a cross country comparison, international society for pharmacoeconomics and outcomes research conference (2004)
Fryback, D.G., Dasbach, E.J., Klein, R., Klein, B.E., Dorn, N., Peterson, K., Martin, P.A.: The Beaver dam health outcomes study: initial catalog of health-state quality factors. Med. Decis. Mak. 13(2), 89–102 (1993)
Cancer statistics-registrations, England, 2001. Series MB1 no.32. London: Office for National Statistics, 2004, http://www.statistics.gov.uk/StatBase/Product.asp?vlnk = 8843&Pos = &ColRank = 1&Rank = 240
Braithwaite, R.S., Chlebowski, R.T., Lau, J., George, S., Hess, R., Col, N.F.: Meta-analysis of vascular and neoplastic events associated with tamoxifen. J. Gen. Intern. Med. 18(11), 937–947 (2003)
Madison, T., Schottenfeld, D., James, S.A. et al.: Endometrial cancer: socioeconomic status and racial/ethnic differences in stage at diagnosis, treatment, and survival. Am. J. Public Health 94, 2104–2111 (2004)
Minelli, L., Stracci, F., Prandini, S., Fusco Moffa, I.: Gynaecological cancers in Umbria (Italy): trends of incidence, mortality and survival, 1978–1998. Eur. J. Obstet. Gynecol. Reprod. Biol. 115, 59–65 (2004)
Fisher, B., Costantino, J.P., Wickerham, D.L., Redmond, C.K., Kavanah, M., Cronin W.M. et al.: Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J. Natl. Cancer Inst. 90(18), 1371–1388 (1998)
Volmink, J.A., Newton, J.N., Hicks, N.R., Sleight, P., Fowler, G.H., Neil, H.A.: Coronary event and case fatality rates in an English population: results of the Oxford myocardial infarction incidence study. The Oxford myocardial infarction incidence study group, Heart 19
Cowie, M.R., Wood, D.A., Coats, A.J.S., Thompson, S.G., Poole-Wilson, P.A., Suresh, V., Sutton, G.C.: Incidence and aetiology of heart failure. A population-based study. Eur. Heart J. 20, 421–428 (1999)
Ward, S., Lloyd Jones, M., Pandor, A., Holmes, M., Ara, R., Ryan, A. et al: Statins for the prevention of coronary events. Technology assessment report commissioned by the HTA programme on behalf of The National Institute for Clinical Excellence, January (2005)
Karnon, J., Brennan, A., Pandor, A., Fowkes, G., Lee, A., Gray, D. et al.: Modelling the long term cost effectiveness of clopidogrel for the secondary prevention of occlusive vascular events in the UK. Curr. Med. Res. Opin. 21, 101–112 (2005)
Marks, D., Wonderling, D., Thorogood, M., Lambert, H., Humphries, S.E., Neil, H.A.W.: Screening for hypercholesterolaemia versus case finding for familial hypercholesterolaemia: a systematic review and cost-effectiveness analysis. Health. Technol. Assess. 4(29), (2000)
British Heart Foundation Statistics Website. Survival after initial diagnosis of heart failure, around 2002, London. Referenced as personal communication with the London Heart Failure Study, http://www.heartstats.org
Stewart, S., Jenkins, A., Buchan, S., McGuire, A., Capewell, S., McMurray, J.J.: The current cost of heart failure to the National Health Service in the UK. Eur J Heart Fail. 4(3), 361–371 (2002)
Karnon, J., Bakhai, A., Brennan, A., Flather, M., Warren, E., Gray, D., Akehurst, R.A.: Cost-utility analysis of clopidogrel in patients with non-ST-segment elevation acute coronary syndromes in the UK. Int. J. Cardiol. (2005)
Kniffin, W.D Jr, Baron, J.A., Barrett, J., Birkmeyer, J.D., Anderson F.A. Jr.: The epidemiology of diagnosed pulmonary embolism and deep venous thrombosis in the elderly. Arch. Intern. Med. 154(8), 861–866 (1994)
Anderson, F.A. Jr, Wheeler, H.B., Goldberg, R.J., Hosmer. D.W., Patwardhan, N.A., Jovanovic, B., Forcier, A., Dalen, J.E.: A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study
Silverstein, M.D., Heit, J.A., Mohr, D.N., Petterson, T.M., O’Fallon, W.M., Melton L.J. III.: Trends in the incidence of deep vein thrombosis and pulmonary embolism. A 25-year population-based study. Arch. Intern. Med. 158, 585–593 (1998)
Locker, G.Y.: on behalf of the ATAC Trialists’ Group, Cost-utility analysis of anastrozole versus tamoxifen as primary adjuvant therapy in postmenopausal women with early breast cancer from a US healthcare system perspective: the 5-year completed treatment analysis of the ATAC (‘Arimidex’, Tamoxifen Alone or in Combination) trial, poster 2085, 27th San Antonio Breast Cancer Symposium (2004)
Rocchi, A., Verma, S.: Anastrozole is cost-effective vs tamoxifen as initial adjuvant therapy in early breast cancer: Canadian perspectives on the ATAC completed-treatment analysis, Support Care Cancer. 5 April 2006 [Epub ahead of print]
Hillner, B.E.: Benefit and projected cost-effectiveness of anastrozole versus tamoxifen as initial adjuvant therapy for patients with early-stage estrogen receptor-positive breast cancer. Cancer 101(6), 1311–1322 (2004)
Acknowledgments
This study was funded by Novartis Pharmaceuticals Corporation, East Hanover, NJ. We are particularly grateful for the advice provided by Natalie Papo, Satyin Kaura, and Francois DiTrapani. This research was conducted independently of the BIG 1-98 Steering Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karnon, J., Delea, T. & Barghout, V. Cost utility analysis of early adjuvant letrozole or anastrozole versus tamoxifen in postmenopausal women with early invasive breast cancer: the UK perspective. Eur J Health Econ 9, 171–183 (2008). https://doi.org/10.1007/s10198-007-0058-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10198-007-0058-1