Abstract
Long tail feathers of the barn swallow Hirundo rustica are a classic example of an intersexually selected trait, but previous aerodynamic analyses indicate that the tail feather is only 10–12 mm longer than the aerodynamic optimum even in the nominate subspecies with long tails. Here, by experimentally shortening female tail length, we studied the feeding cost of long tail feathers in Japanese barn swallows, Hirundo rustica gutturalis, which have ca. 10 mm shorter tails than the nominate subspecies. Female feeding rate was explained by the interaction between treatment and original female tail length: feeding rate decreased with decreasing original female tail length in control, but not in tail-shortened females. Because the interaction term was far from significant in the analysis of female incubation investment, the observed pattern would be specific to feeding rate, which is greatly affected by the aerodynamic properties associated with tail length. Differential allocation of paternal feeding investment was not observed in the current data set. Long tails would be costly at least in short-tailed females, supporting differential costs of ornamentation as predicted by sexual selection theory. Female outermost tail feathers are costly ornamentation in short-tailed Japanese barn swallows.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long outermost tail feathers of the barn swallow Hirundo rustica are a classic example of an intersexually selected trait (Andersson 1994). Longer tailed males have reproductive advantages due to social and extra-pair mate choice (reviewed in Møller 1994). However, it has also been suggested that long tails have aerodynamic advantages (e.g. high manoeuvrability: Norberg 1994; Evans 1998; Buchanan and Evans 2000). For this reason, the cost of ornamentation has attracted keen attention in the last two decades because the cost function may clarify the relative importance of sexual and viability selection on the evolution of long tails (e.g. Buchanan and Evans 2000; Bro-Jørgensen et al. 2007; Cuervo and de Ayala 2014).
A series of manipulative experiments suggest that outermost tail feathers of European barn swallows, H. r. rustica, are 10–12 mm longer than the aerodynamic optimum (Evans 1998; Buchanan and Evans 2000; Rowe et al. 2001). Based on these studies, one may argue that sexually selected component of tail length is only a small portion of the tail feather (e.g. Rowe et al. 2001). However, this argument is problematic, because it is unclear whether the estimated aerodynamic cost reflects fitness cost (e.g. Møller and Barbosa 2001), and because actual cost of long tails would be partially compensated for by the evolution of cost-reducing traits (e.g. Balmford et al. 1994; Møller 1996; Tubaro 2003; also see Husak et al. 2015 for general discussion). As Norberg (1994) already noted, experimental manipulation of tail length upsets an original co-adapted character set, and thus may not reveal the aerodynamic function of long tails, limiting the validity of the finding above. In fact, recent phylogenetic studies indicate that the cost of tail length increased linearly with tail length in highly aerial species (i.e. swallows and swifts: Hasegawa et al. 2016a; Hasegawa and Arai 2017a, 2018), indicating no or few aerodynamic advantages of long tails on an evolutionary timescale. Because flight speed decreases with increasing tail length, long tails might have a selective disadvantage during foraging (also see Hasegawa and Arai 2017b for a natural experiment in the Pacific swallow H. tahitica). It is, therefore, still controversial whether the cost of ornamentation, estimated by aerodynamic property in European subspecies, can explain the evolution of long tails.
Another approach to study the cost of ornamentation is to compare provisioning rate between treatments with experimentally manipulated tail length in females with short tails (Matyjasiak et al. 1999). Although male tail length affects their own feeding rate and their mates’ feeding rate via differential allocation and reproductive compensation (e.g. see Witte 1995; reviewed in Sheldon 2000), such a confounding effect would be of minor importance when manipulating female ornaments (due to weaker sexual selection on female ornaments: Matyjasiak et al. 1999). Although previous studies reported that long tails are costly at least for feeding their young in species with short tails (e.g. sand martins; Matyjasiak et al. 1999, 2000; also see Matyjasiak et al. 2004, 2009 for the effect of tail shape), differential foraging strategies might explain the difference between these birds and barn swallows (e.g. barn swallows fly at lower altitudes with higher speeds, less gliding, and perform more turns, differing from sand martins: Turner and Rose 1994).
Japanese barn swallows (subspecies: H. r. gutturalis) would be a suitable system for studying the cost of ornamentation. This subspecies, like those breeding in the North America, has ca. 10 mm shorter tails than the nominate subspecies, in which previous experiments were conducted (see above; Hasegawa et al. 2010, 2017; reviewed in Turner 2006; Hasegawa 2018). Based on the above aerodynamic studies of the European subspecies, some authors have suggested that the tail length of these subspecies should be closer to the aerodynamic optimum (hereafter referred to as the "optimum" hypothesis), perhaps due to a reduction in sexual selection (e.g. Safran and McGraw 2004). However, there are several differences between subspecies, including morphological traits (e.g. shorter wings: Hasegawa et al. 2010; reviewed in Turner 2006) and thus extrapolating the findings from one population to others might be problematic. Rather, long tail would be costly even in this short-tailed subspecies due to sexual selection for long tails (Hasegawa et al. 2017; also see Hasegawa and Arai 2017c) and small cost-reducing traits (see above), particularly in females with originally short tails (hereafter referred to as the "handicap" hypothesis).
By experimental shortening of female tail length in Japanese barn swallows, we investigated the cost of the ornamentation. As in other subspecies (see above), the provisioning rate (feeding rate, hereafter) of parents reflects the flight ability in our population (Tajima and Nakamura 2003). If the optimum hypothesis, which proposes that tail lengths in these subspecies are close to the aerodynamic optimum (i.e. < 10 mm; see above), is applicable, > 10 mm tail shortening would reduce the feeding rate of females. Alternatively, if the handicap hypothesis, which proposes that long tails are costly under sexual selection, is applicable, tail shortening would increase the feeding rate of females, particularly those with originally short tails, because the same amount of ornamentation is costlier to low-quality individuals (i.e. females with originally short tails; e.g. see Matyjasiak et al. 2000). Because a previous manipulative experiment found no detectable differential allocation at least during the incubation period (Hasegawa et al. 2018), we predicted no differential allocation of male parental care during provisioning as well. Only female feeding rate would be affected by the treatment or its interaction with original female tail length.
Material and methods
Study site
This study was conducted during the early breeding season (30 April–30 June) in 2017 and 2018 in a residential area of Joetsu City, Niigata Prefecture, Japan (37°07′N, 138°15′E). Swallows sparsely nest there under the eaves of covered sidewalks (see Tajima and Nakamura 2003). Detailed methodology was written elsewhere (Hasegawa et al. 2018). In short, we inspected nests every third day to record laying date and clutch size. From 10 days after the onset of incubation, we inspected nests every day to estimate hatching dates.
Adults were captured in sweep nets while roosting at night during their incubation period. Each bird was provided with a numbered aluminium ring and a unique combination of two or three half-sized coloured rings, which were made from plastic rings (AC Hughes, Middlesex, UK). The sex of each individual was determined by the presence (female) or absence (male) of an incubation patch (Turner 2006). We did not determine age in the study sample, because we captured no birds before 2017 and our sample included only a small number of recaptured birds in 2018.
At capture, we measured wing length, tarsus length, body mass, and tail length. The measurement of tail length, estimated from the right outermost tail feather to the nearest 0.01 mm, was found to be highly repeatable (repeatability > 0.99: see Hasegawa et al. 2018).
Experimental manipulation
Female swallows were assigned randomly either to a group in which the outermost tail feathers were shortened (shortened treatment), or a group in which tail feathers would not be shortened (control). In the first group of females, the two outermost tail feathers (streamers) were reduced in length by an average of 11.37 mm (SE = 1.10, range 10.21–13.50) and trimmed at the distant end (see fig. S1 in Hasegawa et al. 2018). We chose 11 mm because this length corresponds to the aerodynamically costly part in female tails (10–12 mm; e.g. Buchanan and Evans 2000; Rowe et al. 2001; Cuervo and de Ayala 2014). In the control group, the distal ends of the outermost tail feathers were trimmed without substantially reducing their length (although trimming might slightly reduce tail length). We did not include a tail elongation treatment, because cut and glue may disturb the Norberg mechanism (Evans and Thomas 1997). We obtained feeding rates for seven tail-shortened females and ten control females (note that we also observed incubation attentiveness as well; see Hasegawa et al. 2018; note that we avoided to use the same individuals in the two study years). Due to the small sample size in each study year (2017: ncontrol = 6, nshortened = 4; 2018: ncontrol = 4, nshortened = 3), we pooled the data for both years. There was no significant difference in original tail length between the two study years (2017: 79.20 ± 1.99 mm; 2018: 80.28 ± 2.06 mm; t = 0.41, P = 0.69) or treatments (control: 79.88 ± 1.35 mm; tail-shortened: 79. 40 ± 2.52 mm; t = − 0.14, P = 0.89; note that this tail length is ca. 10 mm shorter than those in experiments studying the aerodynamic properties of the European subspecies, e.g. mean = 89.55 mm; Rowe et al. 2001).
Nest observations
Nests were observed at the first broods only when the birds were easy to identify. Video camera recorders (GZ-E265; JVC, Tokyo, Japan) were set up ca. 2–3 m from the nests and did not appear to disturb the birds. Each nest was observed for 60 min only once, i.e. when the nestlings were 12 days old. Observations were made in the afternoon in 2017 and the morning in 2018. Unfortunately, we could not identify prey species, which were not visible in the recorded video. We instead recorded the numbers of male and female feeding visits to the nests per 60 min (male and female feeding rates, hereafter). We also recorded the total numbers of parental feeding visits to the nests per 60 min (i.e. the sum of male and female feeding rates: hereafter the total feeding rate).
Statistics
Feeding rate was analysed using a linear model after log-transformation. The significance of the terms was determined by an F test. In each analysis, we fitted a full model containing all explanatory variables (i.e. treatment, original female tail length, and mates’ feeding rate). Residuals of each model did not significantly deviate from a normal distribution (Shapiro–Wilks test, W > 0.91, P > 0.10). Wing length, tarsus length, body mass, laying date, clutch size, and hatching date were not included in the model due to the small sample size. However, they were not significant when added to the models of male (F < 3.66, P > 0.08) and female feeding rates (F < 1.31, P > 0.28), and thus would not confound the results. Regarding total feeding rate, female wing length (but not others) was significant when added to the model (wing length: coefficient ± SE = 0.09 ± 0.03, F = 6.01, P = 0.03; other five variables: F < 1.14, P > 0.30). However, wing length did not remain significant after Bonferroni correction (α = 0.05/6 = 0.008; note that we had no a priori prediction regarding this variable), and the two main variables, treatment and original female tail length, remained nonsignificant when including wing length as a covariate. Thus, whether including this variable or not did not change our main conclusion. All data analyses were performed using the R statistical package (ver. 3.3.1; R Core Team 2016).
Results
Male feeding rate increased with mates’ feeding rate but was not explained by treatment or original female tail length (Table 1; Fig. 1). The interaction between treatment and original female tail length was not significant when added to the model (F = 1.89, P = 0.19, Max VIF = 4.54).
Tail-shortened females and control females did not differ in a male feeding rate, b female feeding rate, or c total feeding rate in Japanese barn swallows. The bar in each boxplot indicates the median feeding rate, and the box shows the first and third quartiles of the data. Whiskers range from the lowest to the highest data points within 1.5 × the interquartile range of the lower and upper quartiles, respectively. The data points beyond the range of the whiskers were outliers and are plotted with circles
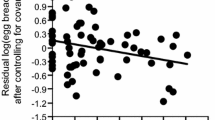
Female feeding rate increased with mates’ feeding rate and original female tail length, but was not explained by treatment (Table 1; Fig. 1). However, the interaction between treatment and original female tail length significantly explained female feeding rate when added to the model (F = 7.99, P = 0.01, Max VIF = 2.69): female feeding rate increased with original female tail length in controls (slope ± SE = 0.10 ± 0.03, t = 4.01, P = 0.002), but not in tail-shortened females (slope ± SE = 0.01 ± 0.02, t = 0.49, P = 0.63; Fig. 2). The linear model predicted that the mean difference in female feeding rate between tail-shortened females and controls was significantly positive in females with originally shorter tails (75 mm, which corresponds to the mean – SD in this population: mean ± SE = 0.56 ± 0.22, t = 2.55, P = 0.025), but was non-significantly negative in females with originally longer tails (85 mm, which corresponds to the mean + SD in this population: − 0.36 ± 0.24, t = − 1.47, P = 0.17).
Relationship between residual log(female feeding rate) after controlling for their mates’ feeding rate in relation to original female tail length and treatment in Japanese barn swallows. Green filled circles (and lines) indicate control females (and a simple regression line based on them) while blue open circles (and lines) indicate tail-shortened females (and a simple regression line based on them). Mean (± SD) female tail length is 79.64 (± 5.20) in the current data set. See text for a formal statistics
Total feeding rate was not significantly explained by either treatment or original female tail length (Table 1; Fig. 1). The interaction between treatment and original female tail length was not significant when added to the model (F = 1.84, P = 0.20, Max VIF = 2.64).
Discussion
One of our findings, i.e. that tail shortening has no detectable effect on the mean feeding rates of females (Fig. 1), seems inconsistent with the optimal and handicap hypotheses. Both of these hypotheses predict that tail shortening affects the mean feeding rates of females. The simple explanation that our manipulation (11 mm tail shortening) is insufficient to affect aerodynamic properties is unlikely, because previous experiments in the European subspecies showed that a similar degree of manipulation affects aerodynamic properties (e.g. Buchanan and Evans 2000; also see Cuervo and Ayala 2014 for the effect of manipulation on physiological properties) and because we observed differential effects of tail shortening, depending on the original tail length of the female (Fig. 2). We discussed other explanations below.
A possible explanation for the observed pattern is that females adjusted their current reproductive investment based on their ornamentation, which is not accounted for in our predictions. For example, long-tailed females may increase their investment in the current brood due to the perceived high reproductive value of that brood compared to future ones (Hasegawa et al. 2016b, 2018). The relatively low intercept of the regression line in tail-shortened females might be due to the female reproductive strategy. Consistent with this perspective, tail-shortened females had a slightly lower reproductive investment than the control females during incubation (Hasegawa et al. 2018, unpublished data), which might mask the subtle effects of tail shortening on aerodynamic properties (see Cuervo and Møller 2006 for the context-dependent effect of tail manipulation on the feeding rate). In fact, tail shortening had a significant positive effect on the female provisioning rate (Table S1; Fig. S1) after controlling for low reproductive investment based on differential nest attentiveness (assuming that females adjusted their reproductive investment similarly during both incubation and provisioning; note that ornamentation affects physiological state, which would then affect their parental behaviour: e.g. Safran et al. 2008; Vitousek et al. 2013).
The observation of a significant interaction term is still consistent with the handicap hypothesis (but not with the optimal hypothesis), as tail shortening increased the feeding rates of females with originally short tails (note that the optimal hypothesis did not predict high feeding rates in tail-shortened females compared with controls, but predicted that deviation from the current “optimal” tail length should decrease feeding rates). The handicap hypothesis predicts that high-quality individuals with originally longer tails cope better with ornamentation, and thus may show no detectable cost of ornamentation, sometimes causing the crossover of regression lines between treatments in manipulative experiments (e.g. Matyjasiak et al. 2000). Because the interaction between treatment and original female tail length was far from significant in incubation investment (Hasegawa et al. 2018; Table S1), the interaction term detected herein would be specific to feeding rate, which would be greatly affected by aerodynamic properties.
Another possible explanation for the observed pattern is the influence of the mates’ contributions. Female feeding rate might be affected by their mates’ contribution to feeding (e.g. see Witte 1995; reviewed in Sheldon 2000). However, this would have minor importance in the current study, because females do not compensate for reduced male feeding rate in this population (at least when males were handicapped: Tajima and Nakamura 2003) and because male feeding rate was not significantly affected by treatment or its interaction with original female tail length. This latter finding is inconsistent with the differential allocation hypothesis, which predicts that males adjust reproductive investment according to their mates’ ornamentation (reviewed in Sheldon 2000), supporting previous findings focusing on incubation attentiveness (Hasegawa et al. 2018).
A caveat of the current study is that we used the feeding rate as the sole measure of provisioning effort. This can be justified by previous studies showing the effect of tail manipulation on feeding rate (e.g. de Lope and Møller 1993; Matyjasiak et al. 1999; Cuervo and Møller 2006; also see Tajima and Nakamura 2003 for reduced feeding rate of handicapped swallows). However, it is plausible that unmeasured foraging aspects, such as prey size, might confound our measure of provisioning effort, as individuals with elongated tails in fact captured many smaller prey items, at least in the case of male European barn swallows (e.g. Møller 1989; Møller and de Lope 1994; Møller et al. 1995; also see Nudds and Spencer 2004 for differential foraging strategy coupled with tail length), obscuring the relationship between experimental manipulation and feeding rate. This possibility alone cannot explain the differential effect of tail shortening depending on the original female tail length, but future studies should concern about both the feeding rate and prey size to account for the total amount of food provisioned.
Unfortunately, we could not clarify whether the tail lengths of these subspecies are closer to the aerodynamic optimum compared to the nominate subspecies, but we found that female outermost tail feathers remain a costly ornamentation, honestly signalling their quality, even in this short-tailed subspecies. This is consistent with the previous studies indicating that long-tailed females are higher quality than short-tailed females (e.g. Hasegawa et al. 2016b, 2017; reviewed in Turner 2006). It remains to be shown whether this quality indicator is, in fact, sexually selected in females, or simply a non-functional copy of a functional male quality indicator via an intersexual genetic correlation (reviewed in Amundsen and Pärn 2006).
Data availability
The datasets analysed during the current study are not publicly available due to ongoing other studies but are available from the corresponding author on reasonable request.
Change history
31 December 2020
The original article can be found online.
References
Amundsen T, Pärn H (2006) Female coloration: review of functional and nonfunctional hypotheses. In: Hill GE, McGraw KJ (eds) Bird coloration. Vol II. Function and evolution. Harvard University Press, Cambridge, pp 280–345
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Balmford A, Jones IL, Thomas ALR (1994) How to compensate for costly sexually selected tails: the origin of sexually dimorphic wings in long-tailed birds. Evolution 48:1062–1070
Bro-Jørgensen J, Johnstone RA, Evans MR (2007) Uninformative exaggeration of male sexual ornaments in barn swallows. Curr Biol 17:850–855
Buchanan KL, Evans MR (2000) The effect of tail streamer length on aerodynamic performance in the barn swallow. Behav Ecol 11:228–238
Cuervo JJ, de Ayala RM (2014) Effects of experimental tail shortening on the phenotypic condition of barn swallows Hirundo rustica: Implications for tail-length evolution. J Avian Biol 45:345–353
Cuervo JJ, Møller AP (2006) Experimental tail elongation in male barn swallows Hirundo rustica reduces provisioning of young, but only in second broods. Ibis 148:449–458
de Lope F, Møller AP (1993) Female reproductive effort depends on the degree of ornamentation of their mates. Evolution 47:1152–1160
Evans MR (1998) Selection on swallow tail streamers. Nature 394:233–234
Evans MR, Thomas ALR (1997) Testing the functional significance of tail streamers. Proc R Soc Lond B 264:211–217
Hasegawa M (2018) Sexual selection mechanisms for male plumage ornaments in Japanese Barn Swallows. Ornithol Sci 17:125–134
Hasegawa M, Arai E (2017a) Egg size decreases with increasing female tail fork depth in family Hirundinidae. Evol Ecol 31:559–569
Hasegawa M, Arai E (2017b) Natural selection on wing and tail morphology in the Pacific Swallow. J Ornithol 158:851–858
Hasegawa M, Arai E (2017c) Negative interplay of tail and throat ornaments at pair formation in male barn swallows. Behaviour 154:835–851
Hasegawa M, Arai E (2018) Convergent evolution of the tradeoff between egg size and tail fork depth in swallows and swifts. J Avian Biol 49:e01684
Hasegawa M, Arai E, Watanabe M, Nakamura M (2010) Mating advantage of multiple male ornaments in the Barn Swallow Hirundo rustica gutturalis. Ornithol Sci 9:141–148
Hasegawa M, Arai E, Kutsukake N (2016a) Evolution of tail fork depth in genus Hirundo. Ecol Evol 6:851–858
Hasegawa M, Arai E, Ito S, Wakamatsu K (2016b) High brood patch temperature of less colourful, less pheomelanic female Barn Swallows. Ibis 158:808–820
Hasegawa M, Arai E, Watanabe M, Nakamura M (2017) Reproductive advantage of multiple female ornaments in the Asian Barn Swallow Hirundo rustica gutturalis. J Ornithol 158:517–532
Hasegawa M, Arai E, Nakamura M (2018) Experimental manipulation of female tail length did not cause differential allocation by males in the barn swallow. Ethology 124:113–121
Husak JF, Henningsen JP, Vanhooydonck B, Irschick DJ (2015) A performance-based approach to studying costs of reliable signals. In: Irschick DJ, Briffa M, Podos J (eds) Animal signaling and function: an integrative approach. Wiley Blackwell, Hoboken, pp 47–74
Matyjasiak P, Jabłoński PG, Olejniczak I, Boniecki PI, Lee S-D (1999) Foraging cost of a long tail ornament: an experiment with sand martin females. Ethology 105:521–530
Matyjasiak P, Jabłoński PG, Olejniczak I, Boniecki P (2000) Imitating the initial evolutionary stage of a tail ornament. Evolution 54:704–711
Matyjasiak P, Matyjasiak J, de Lope F, Møller AP (2004) Vane emargination of outer tail feathers improves flight manoeuvrability in streamer-less hirundines, Hirundinidae. Proc R Soc Lond B 271:1831–1838
Matyjasiak P, Marzal A, Navarro C, de Lope F, Møller AP (2009) Fine morphology of experimental tail streamers and flight manoeuvrability in the house martin Delichon urbica. Funct Ecol 23:389–396
Møller AP (1989) Viability costs of male tail ornaments in a swallow. Nature 339:132–135
Møller AP (1994) Sexual selection and the Barn Swallow. Oxford University Press, Oxford
Møller AP (1996) The cost of secondary sexual characters and the evolution of cost-reducing traits. Ibis 138:112–119
Møller AP, Barbosa A (2001) Flight, fitness and sexual selection. Behav Ecol 12:511–512
Møller AP, de Lope F (1994) Differential costs of a secondary sexual character: an experimental test of the handicap principle. Evolution 48:1676–1683
Møller AP, de Lope F, Lopez Caballero JM (1995) Foraging costs of a tail ornament: experimental evidence from two populations of barn swallows Hirundo rustica with different degrees of sexual size dimorphism. Behav Ecol Sociobiol 37:289–295
Norberg RÅ (1994) Swallow tail streamer is a mechanical device for self-deflection of tail leading edge enhancing aerodynamic efficiency and flight manoeuvrability. Proc R Soc Lond B 257:227–233
Nudds RL, Spencer A (2004) Daily energy expenditure of male barn swallows correlates with tail streamer length: handicap-mediated foraging strategies. Proc R Soc Lond B 271:S160–S163
Rowe LV, Evans MR, Buchanan KL (2001) The function and evolution of the tail streamer in hirundines. Behav Ecol 12:157–163
R Core Team (2016) R: a language and environment for statistical computing. R Foundation for statistical computing, Vienna, Austria. https://www.R-project.org/. Accessed 30 Sept 2019
Safran RJ, McGraw KJ (2004) Plumage coloration, not length or symmetry of tail-streamers, is a sexually selected trait in North American barn swallows. Behav Ecol 15:455–461
Safran RJ, Adelman JS, McGraw KJ, Hau M (2008) Sexual signal exaggeration affects physiological state in male barn swallows. Curr Biol 18:R461–R462
Sheldon BC (2000) Differential allocation: tests, mechanisms and implications. Trends Ecol Evol 15:397–402
Tajima K, Nakamura M (2003) Response to manipulation of partner contribution: a handicapping experiment in the Barn Swallow. Ornithol Sci 2:65–72
Tubaro PL (2003) A comparative study of aerodynamic function and flexural stiffness of outer tail feathers in birds. J Avian Biol 34:243–250
Turner AK (2006) The Barn Swallow. T & AD Poyser, London
Turner AK, Rose C (1994) A handbook to the Swallows and Martins of the world. Helm, London
Vitousek MN, Stewart RA, Safran RJ (2013) Female plumage colour influences seasonal oxidative damage and testosterone profiles in a songbird. Biol Lett 9:20130539
Witte K (1995) The differential-allocation hypothesis: does the evidence support it? Evolution 49:1289–1290
Acknowledgements
We are grateful to the residents of Joetsu City for their kind support and assistance. We also thank Dr. Nobuyuki Kutsukake, and his lab members in Sokendai. MH was supported by the Research Fellowship of the Japan Society for the Promotion of Science (JSPS, 15J10000) and by JSPS KAKENHI Grant (17K15193).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The permits for the current study including capturing were provided by Niigata Prefecture in Japan (#24 for 2017 and #1 for 2018), following the Wildlife Protection and Hunting Management Law.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised due to a retrospective Open Access order.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Hasegawa, M., Arai, E. & Nakamura, M. Experimental tail shortening affects feeding rate depending on original tail length in female barn swallows Hirundo rustica gutturalis. J Ethol 38, 179–184 (2020). https://doi.org/10.1007/s10164-019-00637-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10164-019-00637-y