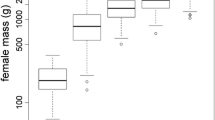
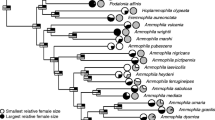
Abstract
Female ornamentation evolves as a nonfunctional copy of functional male ornamentation or through direct selection on female ornamentation. Because females invest many resources in both reproduction and ornamentation, an evolutionary tradeoff between female ornamentation and maternal investment can be predicted, but it has rarely been demonstrated. Using phylogenetic comparative analysis, we studied egg size in relation to female fork depth in the family Hirundinidae, which has a wide range of fork depth (from 0 to >50 mm). Because deeply forked tails are aerodynamically costly, we predicted that species in which females have deeply forked tails suffer reduced egg size due to inefficient foraging during the egg formation period. Egg length was significantly related to nest type but not to female or male tail fork depth, whereas we found a significant negative relationship between egg breadth and female, but not male, tail fork depth. As a consequence, a significant negative relationship was found between egg volume and female, but not male, tail fork depth. Because female and male fork depths were not significantly related to clutch size, clutch size would not compensate the relationship between egg size and fork depth. The current finding supports the hypothesis that female ornamentation trades off with maternal investment.


Similar content being viewed by others
References
Amundsen T, Pärn H (2006) Female coloration: review of functional and nonfunctional hypotheses. In: Hill GE, McGraw KJ (eds) Bird coloration, vol II., Function and evolutionHarvard University Press, Cambridge, pp 280–345
Andersson M (1994) Sexual selection. Princeton University Press, Princeton
Andersson S, Pryke SR, Örnborg J, Lawes MJ, Andersson M (2002) Multiple receivers, multiple ornaments, and a trade-off between agonistic and epigamic signaling in a widowbird. Am Nat 160:683–691
Badyaev AV (1997) Covariation between life history and sexually selected traits: an example with cardueline finches. Oikos 80:128–138
Balmford A, Jones IL, Thomas ALR (1994) How to compensate for costly sexually selected tails: the origin of sexually dimorphic wings in long-tailed birds. Evolution 48:1062–1070
Barbosa A (1999) Tail streamers and flight performance in barn swallows: natural or sexual selection? Ardeola 46:101–109
Bókony V, Liker A (2005) Melanin-based black plumage coloration is related to reproductive investment in cardueline finches. Condor 107:775–787
Bonisoli-Alquati A, Martinelli R, Rubolini D, Saino N (2008) Sex-specific effects of albumen removal and nest environment manipulation on barn swallow nestlings. Ecology 89:2315–2324
Buchanan KL, Evans MR (2000) The effect of tail streamer length on aerodynamic performance in the barn swallow. Behav Ecol 11:228–238
Chenoweth SF, Doughty P, Kokko H (2006) Can non-directional male mating preferences facilitate honest female ornamentation? Ecol Lett 9:179–184
Collias NE (1997) On the origin and evolution of nest building by passerine birds. Condor 99:253–277
Cuervo JJ, de Lope F, Møller AP (1996) The function of long tails in female barn swallows (Hirundo rustica): an experimental study. Behav Ecol 7:132–136
Cuervo JJ, Møller AP, de Lope F (2003) Experimental manipulation of tail length in female barn swallows (Hirundo rustica) affects their future reproductive success. Behav Ecol 14:451–456
Doutrelant C, Gregoire A, Midamegbe A, Lambrechts M, Perret P (2012) Female plumage coloration is sensitive to the cost of reproduction. An experiment in blue tits. J Anim Ecol 81:87–96
Dudley R (2002) Mechanisms and implications of animal flight maneuverability. Integr Comp Biol 42:135–140
Evans MR (1998) Selection on swallow tail streamers. Nature 394:233–234
Evans MR (1999) Reply: length of tail streamers in barn swallows. Nature 397:115–116
Fitzpatrick JW (1985) Form, foraging behavior, and adaptive radiation in the Tyrannidae. Ornithol Monogr 36:447–470
Fitzpatrick S, Berglund A, Rosenqvist G (1995) Ornaments or offspring: costs to reproductive success restrict sexual selection processes. Biol J Linn Soc 55:251–260
Fox J, Weisberg S (2011) An R companion to applied regression, 2nd edn. Sage Publications, Thousand Oaks
Garamszegi LZ, Mundry R (2014) Multimodel-inference in comparative analyses. In: Garamszegi LZ (ed) Modern phylogenetic comparative methods and their application in evolutionary biology: concepts and practice. Springer, New York, pp 305–331
Hails CJ (1982) A comparison of tropical and temperate aerial insect abundance. Biotropica 14:310–313
Hasegawa M, Arai E (2016) Rufous coloration associated with terrestrial locomotion in the adaptive radiation of Malagasy Couas. J Ornithol 157:1115–1118
Hasegawa M, Arai E, Kutsukake N (2016a) Evolution of tail fork depth in genus Hirundo. Ecol Evol 6:851–858
Hasegawa M, Arai E, Watanabe M, Nakamura M (2016b) Reproductive advantage of multiple female ornaments in the Asian Barn Swallow Hirundo rustica gutturalis. J Ornithol. doi:10.1007/s10336-016-1401-z
Hoyt DW (1979) Practical methods of estimating volume and fresh weight of bird eggs. Auk 96:73–77
Johnson LS, Leyhe JE, Werner C (2001) The shape of eggs in different-sized clutches of the House Wren (Troglodytes aedon). Can J Zool 79:1527–1531
Krist M (2011) Egg size and offspring quality: a meta-analysis in birds. Biol Rev 86:692–716
Lande R (1980) Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34:292–305
Loiselle BA, Hoppes WG (1983) Nest predation in insular and mainland lowland rainforest in Panama. Condor 85:93–95
Matyjasiak P, Jablonski PG, Olejniczak I, Boniecki P, Lee S-D (1999) Foraging cost of a long tail ornament: an experiment with sand martin females. Ethology 105:521–530
Matyjasiak P, Jablonski PG, Olejniczak I, Boniecki P (2000) Imitating the initial evolutionary stage of a tail ornament. Evolution 54:704–711
Matyjasiak P, Marzal A, Navarro C, de Lope F, Møller AP (2009) Fine morphology of experimental tail streamers and flight manoeuvrability in the house martin Delichon urbica. Funct Ecol 23:389–396
Møller AP (1989) Viability costs of male tail ornaments in a swallow. Nature 339:132–135
Møller AP (1994) Sexual selection and the barn swallow. Oxford University Press, Oxford
Møller AP (1996) The cost of secondary sexual characters and the evolution of cost-reducing traits. Ibis 138:112–119
Møller AP, Barbosa A (2001) Flight, fitness and sexual selection. Behav Ecol 12:511–512
Møller AP, de Lope F (1994) Differential costs of a secondary sexual character: an experimental test of the handicap principle. Evolution 48:1676–1683
Møller AP, de Lope F, Lopez Caballero JM (1995) Foraging costs of a tail ornament: experimental evidence from two populations of barn swallows Hirundo rustica with different degrees of sexual size dimorphism. Behav Ecol Sociobiol 37:289–295
Morales J, Velando A, Torres R (2009) Fecundity compromises attractiveness when pigments are scarce. Behav Ecol 20:117–123
Mundry R (2014) Statistical issues and assumptions of phylogenetic generalized least squares. In: Garamszegi LZ (ed) Modern phylogenetic comparative methods and their application in evolutionary biology: concepts and practice. Springer, New York, pp 131–153
Norberg RA (1994) Swallow tail streamer is a mechanical device for self deflection of tail leading edge, enhancing aerodynamic efficiency and flight manoeuvrability. Proc R Soc Lond B Biol Sci 257:227–233
Nordeide JT, Kekäläinen J, Janhuhen M, Kortet R (2013) Female ornaments revisited—are they correlated with offspring quality? J Anim Ecol 82:26–38
Paradis E, Claude J, Strimmer K (2004) APE: analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290
Park KJ, Evans MR, Buchanan KL (2000) Assessing the aerodynamic effects of tail elongations in the house martin (Delichon urbica): implications for the initial selection pressures in hirundines. Behav Ecol Sociobiol 48:364–372
Pinheiro J, Bates D, DebRoy S, Sarkar D (2015) Package ‘nlme’. http://cran.r-project.org/web/packages/nlme/nlme.pdf. Accessed 21 Dec 2015
Potti J, Canal D, Serrano D (2013) Lifetime fitness and age-related female ornament signalling: evidence for survival and fecundity selection in the pied flycatcher. J Evol Biol 26:1445–1457
R Development Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. http://www.R-project.org/. Accessed 1 Aug 2015
Rowe LV, Evans MR, Buchanan KL (2001) The function and evolution of the tail streamer in hirundines. Behav Ecol 12:157–163
Rubolini D, Liker A, Garamszegi LZ, Møller AP, Saino N (2015) Using the BirdTree.org website to obtain robust phylogenies for avian comparative studies: a primer. Curr Zool 61:959–965
Saino N, Romano M, Ambrosini R, Ferrari RP, Møller AP (2004) Timing of reproduction and egg quality covary with temperature in the insectivorous barn swallow, Hirundo rustica. Funct Ecol 18:50–57
Sheldon FH, Whittingham LA, Moyle RG, Slikas B, Winkler DW (2005) Phylogeny of swallows (Aves: Hirundinidae) estimated from nuclear and mitochondrial DNA sequences. Mol Phylogenetics Evol 35:254–270
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Thomas ALR (1993) On the aerodynamics of birds’ tails. Philos Trans R Soc Lond B 340:361–380
Tubaro PL (2003) A comparative study of aerodynamic function and flexural stiffness of outer tail feathers in birds. J Avian Biol 34:243–250
Turner AK (1982) Optimal foraging by the swallow (Hirundo rustica L.): prey size selection. Anim Behav 30:862–872
Turner AK (2004) Family Hirundinidae (swallows and martins). In: Del Hoyo J, Elliott A, Christie D (eds) The birds of the world, vol 9. Lynx Edicions, Barcelona, pp 602–685
Turner AK (2006) The barn swallow. T & A D Poyser, London
Turner AK, Rose C (1994) A handbook to the swallows and martins of the world. Christopher Helm, London
Ward S (1996) Energy expenditure of female barn swallows Hirundo rustica during egg formation. Physiol Zool 69:930–951
Ward S, Bryant DM (2006) Barn Swallows Hirundo rustica form eggs mainly from current food intake. J Avian Biol 37:179–189
Winkler DW, Sheldon FH (1993) Evolution of nest construction in swallows (Hirundinidae): a molecular phylogenetic perspective. Proc Natl Acad Sci USA 90:5705–5707
Acknowledgements
We thank Dr Nobuyuki Kutsukake and his lab members at Sokendai (The Graduate University for Advanced Studies) for their kindest advices. We are grateful to Dr Angela Turner for her kindly support on the valuable information on swallows. MH was supported by the Research Fellowship of the Japan Society for the Promotion of Science (JSPS, 15J10000).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hasegawa, M., Arai, E. Egg size decreases with increasing female tail fork depth in family Hirundinidae. Evol Ecol 31, 559–569 (2017). https://doi.org/10.1007/s10682-017-9895-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10682-017-9895-2