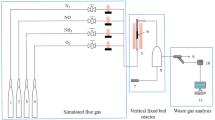
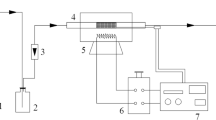
Abstract
Increasing concern about the air pollution caused by sulfur dioxide (SO2) from diesel exhaust has resulted in the improvement of low-temperature desulfurization materials for the combined SO2 trap. In this study, coconut shell activated carbon (AC) is pretreated by nitric acid to prepare MnO2-based activated carbon materials for SO2 removal. The prepared materials are characterized intensively by SEM, TEM, BET, XRD, FTIR, and XPS. The SO2 capture capacity of these materials are measured at low temperature by thermogravimetry, and the SO2 equilibrium adsorption characteristic is also investigated. The results show that the concentrations of nitric acid do not significantly change the textural properties of MnO2-based AC materials. The content of surface-oxygenated groups (carbonyl carbon and transition) initially increases with the HNO3 concentration rising and reaches the maximum value when the HNO3 concentration is 10 mol/L, resulting in the enhancement of the SO2 capture capacity. SO2 capture capacity of MnO2-based activated carbon decreases after regeneration and keeps stable after several cycles of thermal regeneration. The experimental data for SO2 adsorption on MnO2-based AC composite can fit the Freundlich model well in comparison with Langmuir model.









Similar content being viewed by others
References
Osaka Y, Kito T, Kobayashi N et al (2015) Removal of sulfur dioxide from diesel exhaust gases by using dry desulfurization MnO2 filter. Sep Purif Technol 150:80–85
Lee KT, Bhatia S, Mohamed AR (2005) Preparation and characterization of sorbents prepared from ash (waste material) for sulfur dioxide (SO2) removal. J Mater Cycles Waste 7(1):16–23
Hunsinger H, Andersson S (2014) The potential of SO2 for reducing corrosion in WtE plants. J Mater Cycles Waste 16(4):657–664
Limousy L, Mahzoul H, Brilhac JF et al (2003) A study of the regeneration of fresh and aged SO x adsorbers under reducing conditions. Appl Catal B: Environ 45(3):169–179
Limousy L, Mahzoul H, Brilhac JF et al (2003) SO2 sorption on fresh and aged SOx traps. Appl Catal B: Environ 42(3):237–249
Tikhomirov K, Krocher O, Elsener M et al (2006) Manganese based materials for diesel exhaust SO2 traps. Appl Catal B: Environ 67(3–4):160–167
Liu XC, Osaka Y, Huang HY et al., Development of low-temperature desulfurization performance of a MnO2/AC composite for a combined SO2 trap for diesel exhaust. RSC Adv. 2016, 6, (98), 96367–96375
Osaka Y, Kurahara S, Kobayashi N, Hasatani M, Matsuyama A (2015) Study on SO2-absorption behavior of composite materials for DeSO(x) filter from diesel exhaust. Heat Transfer Eng 36(3):325–332
Sohn HY, Han DH (2002) Ca-Mg acetate as dry SO2 sorbent: III. Sulfation of MgO plus CaO. Aiche J 48(12):2985–2991
Zhang D, Ji L, Liu Z et al (2015) Kinetics of thermal regeneration of SO2-captured V2O5/AC. Ind Eng Chem Res 54(38):9289–9295
Cantu M, Lopez-Salinas E, Valente JS (2005) SO x removal by calcined MgAIFe hydrotalcite-like materials: Effect of the chemical composition and the cerium incorporation method. Environ Sci Technol 39(24):9715–9720
Rubio B, Izquierdo MT (2010) Coal fly ash based carbons for SO2 removal from flue gases. Waste Manage 30(7):1341–1347
Tseng HH, Wey MY (2004) Study of SO2 adsorption and thermal regeneration over activated carbon-supported copper oxide catalysts. Carbon 42(11):2269–2278
Liu X, Osaka Y, Huang H et al (2016) Development of high-performance SO2 trap materials in the low-temperature region for diesel exhaust emission control. Sep Purif Technol 162:127–133
Liu X, Osaka Y, Huang H et al (2017) Development of compact MnO2 filter for removal of SO2 from diesel vehicle emission. RSC Adv 7:18500–18507
Liu Y, Qu Y, Guo J et al (2015) Thermal regeneration of manganese supported on activated carbons treated by HNO3 for desulfurization. Energy Fuels 29(3):1931–1940
Zhang G, Li Z, Zheng H et al (2015) Influence of the surface oxygenated groups of activated carbon on preparation of a nano Cu/AC catalyst and heterogeneous catalysis in the oxidative carbonylation of methanol. Appl Catal B: Environ 179:95–105
Xu J, Zhao J, Xu J et al (2014) Influence of surface chemistry of activated carbon on the activity of gold/activated carbon catalyst in acetylene hydrochlorination. Ind Eng Chem Res 53(37):14272–14281
Alegre C, Gálvez ME, Baquedano E et al (2013) Oxygen-functionalized highly mesoporous carbon xerogel based catalysts for direct methanol fuel cell anodes. J Phys Chem C 117(25):13045–13058
Rodrigues EG, Pereira MFR, Chen X et al (2011) Influence of activated carbon surface chemistry on the activity of Au/AC catalysts in glycerol oxidation. J Catal 281(1):119–127
Aguilar C, García R, Soto-Garrido G et al (2003) Catalytic wet air oxidation of aqueous ammonia with activated carbon. Appl Catal B: Environ 46(2):229–237
Yuan A, Zhang Q (2006) A novel hybrid manganese dioxide/activated carbon supercapacitor using lithium hydroxide electrolyte. Electrochem Commun 8(7):1173–1178
Xia Y, Meng L, Jiang Y et al; Zhao M (2015) Facile preparation of MnO2 functionalized baker’s yeast composites and their adsorption mechanism for Cadmium. Chem Eng J 259:927–935
Lu L, Tian H, He J et al., Graphene-MnO2 Hybrid nanostructure as a new catalyst for formaldehyde oxidation. J Phys Chem C 2016, 120, (41), 23660–23668
Xie X, Gao L (2007) Characterization of a manganese dioxide/carbon nanotube composite fabricated using an in situ coating method. Carbon 45(12):2365–2373
Chu HY, Lai QY, Wang L et al (2010) Preparation of MnO2/WMNT composite and MnO2/AB composite by redox deposition method and its comparative study as supercapacitive materials. Ionics 16(3):233–238
Guo JX, Fan L, Peng JF et al (2014) Desulfurization activity of metal oxides blended into walnut shell based activated carbons. J Chem Technol Biotechnol 89(10):1565–1575
Yang L, Jiang X, Yang ZS et al (2015) Effect of MnSO4 on the removal of SO2 by manganese-modified activated coke. Ind Eng Chem Res 54(5):1689–1696
Fan L, Chen J, Guo J et al (2013) Influence of manganese, iron and pyrolusite blending on the physiochemical properties and desulfurization activities of activated carbons from walnut shell. J Anal Appl Pyrol 104:353–360
Shangguan J, Li CH, Miao MQ et al (2008) Surface characterization and SO2 removal activity of activated semi-coke with heat treatment. New Carbon Mater 23(1):37–43
Zuo Y, Yi H, Tang X, Zhao S, Zhang B, Wang Z, Gao F (2015) Study on active coke-based adsorbents for SO2 removal in flue gas. J Chem Technol Biotechnol 90(10):1876–1885
Al-Harahsheh M, Shawabkeh R, Batiha M et al (2014) Sulfur dioxide removal using natural zeolitic tuff. Fuel Process Technol 126(0):249–258
Gupta VK, Ali I, Suhas et al (2003) Equilibrium uptake and sorption dynamics for the removal of a basic dye (basic red) using low-cost adsorbents. J Colloid Interface Sci 265(2):257–264
Acknowledgements
This research was supported by the National Natural Science Foundation of China (NSFC) through International (Regional) Cooperation and Exchange Projects (Grant No. 21550110494), Chinese Academy of Sciences President’s International Fellowship Initiative (Grant No. 2016VTC068), Guangdong Provincial Science and Technology Plan Projects, P. R. China (Grant No. 2016A050502040).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Liu, X., Liu, L., Osaka, Y. et al. Study on desulfurization performance of MnO2-based activated carbon from waste coconut shell for diesel emissions control. J Mater Cycles Waste Manag 20, 1499–1506 (2018). https://doi.org/10.1007/s10163-018-0710-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-018-0710-0