Abstract
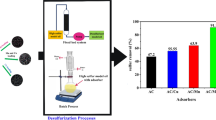
This study reports the usage of chemically impregnated coconut coir waste (CICCW) as a low-cost adsorbent for the desulfurization of feed diesel. The characterization of the developed adsorbent was focused on quantitative analysis (carbon yield %, proximate, ultimate, carbon surface functionalities, BET surface area and porosity distribution, and particle size analysis), qualitative analysis (FTIR), and optical analysis (SEM). Batch experiments with feed diesel having a total sulfur concentration of 2,050 mg L−1 were conducted to optimize the adsorption parameters such as adsorbent dose, temperature, and contact time. The adsorption process shows an optimum dose of 1 g/20 mL, and the equilibrium is attained in 3 h. The adsorption of sulfur onto the adsorbent at optimum temperature 293 K is regulated by external mass transfer (diffusion into mesopores) followed by a steady adsorption phase with intra-particle diffusion in micropores. A Fickian mechanism controls the diffusion of sulfur molecules from the solution onto the surface of the adsorbent. Freundlich adsorption isotherm illustrates the equilibrium adsorption data very well. The negative value of ΔG° (−27.61 kJ mol−1) and ΔS° (−44.56 J K−1 mol−1) indicates the feasibility, spontaneity of the adsorption process and justified the decrease in the randomness of adsorbed sulfur molecules onto the adsorbent surface, respectively. The exhausted CICCW can be effectively regenerated by methanol and reutilized for three adsorption–desorption cycles. The approximate cost of preparation of the adsorbent was USD 10.714 per kg. These results clearly proved the feasibility of the developed low-cost adsorbent (CICCW) as a good candidate for the desulfurization of feed diesel.








Similar content being viewed by others
References
Achmann S, Hagen G, Hammerle M, Malkowsky I, Kiener C, Moos R (2010) Sulfur removal from low-sulfur gasoline and diesel fuel by metal-organic frameworks. Chem Eng Technol 33:275–280
Ahmed Md JK, Ahmaruzzaman M, Reza RA (2014) Lignocellulosic-derived modified agricultural waste: development, characterisation and implementation in sequestering pyridine from aqueous phase. J Colloid Interface Sci 428:222–234
Allen SJ, McKay G, Khader KYH (1989) Intraparticle diffusion of a basic dye during adsorption onto sphagnum peat. Environ Pollut 56:39–50
Babich IV, Moulijn JA (2003) Science and technology of novel processes for deep desulfurization of oil refinery streams: a review. Fuel 82:607–631
Barrett EP, Joyner LG, Halenda PP (1951) The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J Am Chem Soc 73:373–380
Bhattacharya AK, Venkobachar C (1984) Removal of cadmium(II) by low-cost adsorbents. J Environ Eng 110:110–122
Boehm HP (1996) Chemical identification of surface groups. In: Eley DD, Pines H, Weisz PB (eds) Advances in catalyses and related subjects, vol 16. Academic Press, New York, pp 179–274
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Fallah RN, Azizian S (2012) Rapid and facile desulphurization of liquid fuel by carbon nanoparticles dispersed in aqueous phase. Fuel 95:93–96
Fallah RN, Azizian S (2014) Removal of thiophenic compounds from liquid fuel by different modified activated carbon cloths. Fuel 93:45–52
Greg SJ, Sing KSW (1982) Adsorption, surface area and porosity. Academic Press, London
Jagtoyen M, Derbyshire F (1993) Some considerations of the origins of porosity in carbons from chemically activated wood. Carbon 32:1185–1192
Jiang Z, Liu Y, Sun X, Shen Z, Han C, Li C (2003) Adsorption of dibenzothiophene on modified activated carbons for ultra-deep desulfurization of fuel oils. Chin J Catal 24:649–650
Khan NA, Jhung SH (2013) Effect of central metal ions of analogous metal-organic frameworks on the adsorptive removal of benzothiophene from a model fuel. J Hazard Mater 260:1050–1056
Khan SA, Rehman R, Khan MA (1995) Adsorption of Cr(III), Cr(VI) and Ag(I) on Bentonite. Waste Manag 15:271–282
Laredo GC, Vega-Merino PM, Trejo-Zarraga F, Castillo J (2013) Denitrogenation of middle distillates using adsorbent materials towards ULSD production: a review. Fuel Proc Technol 106:21–32
Linsen BG (1970) Physical and chemical aspects of adsorbents and catalyst. Academic Press, London
Murzin D, Salami T (2005) Chemical kinetics. Elsevier, Amsterdam
Nowack KO, Cannon FS, Arora H (1999) Ferric chloride plus GAC for removing TOC. J Am Water Works Assoc 91:65–78
Park JG, Ko CH, Yi KB et al (2008) Reactive adsorption of sulfur compounds in diesel on nickel supported on mesoporous silica. App Catal B Environ 81:244–250
Sarah LG, Kim DT, Alicia MD, Anthony CT, Heather AA (2010) Standardization of Boehm titration. Part I. CO2 expulsion and end point determination. Carbon 48:1252–1261
Sarda KK, Bhandari A, Pant KK, Jain S (2012) Deep desulfurization of diesel fuel by selective adsorption over Ni/Al2O3 and Ni/ZSM-5 extrudates. Fuel 93:86–91
Sing KSW, Everett DH, Haul RAW et al (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl Chem 57:603–619
Song C (2002) Fuel processing for low-temperature and high-temperature fuel cells: challenges, and opportunities for sustainable development in the 21st century. Catal Today 77:17–49
Subhan F, Liu BS (2011) Acidic sites and deep desulfurization performance of nickel supported mesoporous AIMCM-41 sorbent. Chem Eng J 178:69–77
Tein C (1994) Adsorption calculations and modelling. Butterworth-Heinemann, Boston
Triantafyllidis KS, Deliyanni EA (2014) Desulfurization of diesel fuels: adsorption of 4,6-DMDBT on different origin and surface chemistry nanoporous activated carbons. Chem Eng J 236:406–414
Whitehurst DD, Knudsen KG, Wiwel P, Zeuthen P (2000) The influence of trace amounts of nitrogen compounds on the achievement of future sulfur specifications in diesel fuels—Part 2: HDS inhibition studies with real feeds and isolated N-compound fractions. Am Chem Soc Div Petrol Chem 45:367–370
Xiao J, Li Z, Liu B, Xia Q, Yu M (2008) Adsorption of benzothiophene and dibenzothiophene on ion-impregnated activated carbons and ion-exchanged Y zeolites. Energy Fuels 22:3858–3863
Zhao L, Chen Y, Gao J, Chen Y (2010) Desulfurization mechanism of FCC gasoline: a review. Front Chem Eng China 4:314–321
Zhou A, Ma X, Song C (2009) Effects of oxidative modification of carbon surface on the adsorption of sulfur compounds in diesel fuel. Appl Catal B Environ 87:190–199
Acknowledgments
The authors are thankful to Director, National Institute of Technology Silchar and Indian Oil Corporation Limited. (IOCL), Guwahati refinery for providing laboratory facilities for accomplishing the work. Special thanks to Dr. Ashutosh Mishra (SQC Officer, IOCL) for helping in XRF analysis of feed diesel sample for sulfur concentration. The authors are also thankful to anonymous reviewers for their valuable comments in upgrading the quality of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahmed, M.J.K., Ahmaruzzaman, M. Adsorptive desulfurization of feed diesel using chemically impregnated coconut coir waste. Int. J. Environ. Sci. Technol. 12, 2847–2856 (2015). https://doi.org/10.1007/s13762-014-0654-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13762-014-0654-4