Abstract
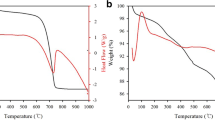
In this paper, the effects of chloride salt (MgCl2, CaCl2 or NaCl) addition on the thermal decomposition of five inorganic mercury compounds (HgCl2, HgS, Hg(NO3)2·H2O, HgO, and HgSO4) were investigated by thermogravimetric analysis. Mercury-contaminated soil samples collected from Inner Mongolia were used to verify the results. The desorption temperatures of the mercury compounds increased in the following order: HgCl = HgCl2 < HgS < Hg(NO3)2·H2O < HgO < HgSO4. Among the chloride salts, MgCl2 had the greatest effect on thermal desorption of the mercury compounds, with the greatest reduction in the initial temperature of thermal desorption. After MgCl2 treatment, the mercury removal rates for the soil were 65.67–81.35 % (sample A), 70.74–84.91 % (sample B), and 69.08 % (sample C). The increase in the mercury removal rate for sample C with addition of MgCl2 was particularly large (34.96–69.08 %). X-Ray diffraction analysis of white crystals from the thermal desorption with MgCl2 indicated that MgCl2 promoted conversion of the mercury compounds in the soil to mercury(II) chloride and dimercury dichloride. This transformation is beneficial for applying thermal desorption to remedy mercury-contaminated soils and treat of mercury containing waste.






Similar content being viewed by others
References
Rong Q, Zeqin D, Junfang Z et al (2013) Overviews on pollution and restoration measures of mercury in contaminated soil. Chin J Environ Protect Technol 19(3):21–26
Li P, Feng XB, Qiu GL et al (2009) Mercury pollution in Asia: a review of the contaminated sites. J Hazard Mater 168(2):591–601
Lusilao-Makiese JG et al (2013) The impact of post gold mining on mercury pollution in the West Rand region, Gauteng, South Africa. J Geochem Explor 134:111–119
Pirrone N, Cinnirella S, Feng X et al (2010) Global mercury emissions to the atmosphere from anthropogenic and natural sources. Atmos Chem Phys 10(13):5951–5964
Henriques B, Rodrigues SM, Coelho C et al (2013) Risks associated with the transfer of toxic organo-metallic mercury from soils into the terrestrial feed chain. Environ Int 59:408–417
Fantozzi L, Ferrara R, Dini F et al (2013) Study on the reduction of atmospheric mercury emissions from mine waste enriched soils through native grass cover in the Mt. Amiata region of Italy. Environ Res 125:69–74
Tipping E, Lofts S, Hooper H et al (2010) Critical limits for Hg(II) in soils, derived from chronic toxicity data. Environ Pollut 158(7):2465–2471
Busto Y, Cabrera X, Tack FMG et al (2011) Potential of thermal treatment for decontamination of mercury containing wastes from chlor-alkali industry. J Hazard Mater 186(1):114–118
Rodríguez O, Padilla I, Tayibi H et al (2012) Concerns on liquid mercury and mercury-containing wastes: a review of the treatment technologies for the safe storage. J Environ Manag 101:197–205
Information on http://nepis.epa.gov/Adobe/PDF/30004RNL.pdf
de Jing Y, Zhao SP, He ZL (2010) A review on the studies on mercury absorption-desorption behavior in the soil. Chin J Soil Sci 5:1270–1274
Hojdová M, Navrátil T, Rohovec J et al (2009) Mercury distribution and speciation in soils affected by historic mercury mining. Water Air Soil Pollut 200(1–4):89–99
Wang J, Feng X, Anderson CWN et al (2012) Remediation of mercury contaminated sites—a review. J Hazard Mater 221:1–18
Chang TC, Yen JH (2006) On-site mercury-contaminated soils remediation by using thermal desorption technology. J Hazard Mater 128(2):208–217
Navarro A, Cañadas I, Martinez D et al (2009) Application of solar thermal desorption to remediation of mercury-contaminated soils. Sol Energy 83(8):1405–1414
Huang Y-T, Hseu Z-Y, Hsi H-C (2011) Influences of thermal decontamination on mercury removal, soil properties, and repartitioning of coexisting heavy metals. Chemosphere 84(9):1244–1249
Chang TC, You SJ, Yu BS et al (2009) Treating high-mercury-containing lamps using full-scale thermal desorption technology. J Hazard Mater 162(2):967–972
Yongguang YIN, Yanbin L et al (2011) Environmental photo-chemistry of mercury. Chin J Environ Chem 30(1):84–89
Kabata-Pendias A (2010) Trace elements in soils and plants, 4th edn. CRC Press, Boca Raton
Gabriel MC, Williamson DG (2004) Principal biogeochemical factors affecting the speciation and transport of mercury through the terrestrial environment. Environ Geochem Health 26(3–4):421–434
Meng Y, Wang S (2012) Study on mercury re-emissions during fly ash utilization. Chin J Environ Sci 33(9):2993–2999
Eichholz Geoffrey G, Frank Petelka M, Kury RL (1988) Migration of elemental mercury through soil from simulated burial sites. Water Res 22(1):15–20
Xu J, Kleja DB, Biester H et al (2014) Influence of particle size distribution, organic carbon, pH and chlorides on washing of mercury contaminated soil. Chemosphere 109:99–105
Lei MK, Peng F, Cao PL et al (2013) Simultaneous determination of arsenic and mercury in tungsten ore by microwave digestion-hydride generation-atomic fluorescence spectrometry. Metall Anal 4:008
Guo W et al (2011) Application of ion molecule reaction to eliminate WO interference on mercury determination in soil and sediment samples by ICP-MS. J Anal Atom Spectr 26(6):1198–1203
Abdalla AA, Smith RE (2013) Determination of mercury in ayurvedic dietary supplements that are not rasa shastra using the hydra-C direct mercury analyzer. Int J Anal Chem 27:71–83
López-Antón MA et al (2012) Analytical methods for mercury analysis in coal and coal combustion by-products. Int J Coal Geol 94:44–53
Chen H et al (2011) Waveband selection for NIR spectroscopy analysis of soil organic matter based on SG smoothing and MWPLS methods. Chemom Intell Lab Syst 107(1):139–146
Slopiecka K, Bartocci P, Fantozzi F (2012) Thermogravimetric analysis and kinetic study of poplar wood pyrolysis. Appl Energy 97:491–497
Pei LSNLL, Yingchun QBY (2011) Oxidation absorption of mercury from flue gas by KMnO4. Chin J Environ Eng 7:035
Rumayor M et al (2013) Mercury compounds characterization by thermal desorption. Talanta 114:318–322
Acknowledgments
This work was supported by the Public Welfare of Environmental Protection Industry Scientific Research Fund (Project No. 201309020), the Key Laboratory for Solid Waste Management and Environment Safety Open Fund (Grant No. SWMES 2013-11) and the Central Public-interest Scientific Institution Basal Research Fund of China (Project No. gyk1061301).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, T., Wang, X., Yang, X. et al. Thermogravimetric and XRD study of the effects of chloride salts on the thermal decomposition of mercury compounds. J Mater Cycles Waste Manag 19, 712–717 (2017). https://doi.org/10.1007/s10163-016-0468-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10163-016-0468-1