Abstract
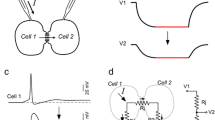
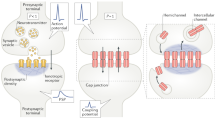
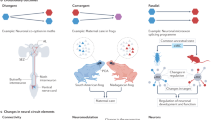
The outstanding behavioural capacity of cephalopods is underpinned by a highly sophisticated nervous system anatomy and neural mechanisms that often differ significantly from similarly complex systems in vertebrates and insects. Cephalopods exhibit considerable behavioural flexibility and adaptability, and it might be expected that this should be supported by evident cellular and synaptic plasticity. Here, we review what little is known of the cellular mechanisms that underlie plasticity in cephalopods, particularly from the point of view of synaptic function. We conclude that cephalopods utilise short-, medium-, and long-term plasticity mechanisms that are superficially similar to those so far described in vertebrate and insect synapses. These mechanisms, however, often differ significantly from those in other animals at the biophysical level and are deployed not just in the central nervous system, but also to a limited extent in the peripheral nervous system and neuromuscular junctions.





Similar content being viewed by others
References
Abbott NJ, Lieberman EM, Pichon Y, Hassan S, Larmet Y (1988) Periaxonal K+ regulation in the small squid Alloteuthis. Studies on isolated and in situ axons. Biophys J 53:275–279
Baldwin MJ (1896) A new factor in evolution. Am Nat 30:441–451
Benjamin PR, Kemenes G, Kemenes I (2008) Non-synaptic neuronal mechanisms of learning and memory in gastropod molluscs. Front Biosci 13:4051–4057
Bliss TV, Lomo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232:331–356
Bloedel J, Gage PW, Llinás R, Quastel DM (1966) Transmitter release at the squid giant synapse in the presence of tetrodotoxin. Nature 212:49–50
Bone Q, Howarth J (1980) The role of l-glutamate in neuromuscular-transmission in some mollusks. J Mar Biol Assoc UK 60:619–626
Bone Q, Brown E, Travers G (1994) On the respiratory flow in the cuttlefish Sepia officinalis. J Exp Biol 194:153–165
Bone Q, Brown ER, Usher M (1995) The structure and physiology of cephalopod muscle fibres. In: Abbott NJ, Williamson R, Maddock L (eds) Cephalopod neurobiology: neuroscience studies in squid, octopus and cuttlefish. Oxford University Press, London, pp 301–329
Brown E (1993) K+ accumulation around the giant axon of the squid: comparison of electrical and morphological measurements. Jpn J Physiol 43:S279
Brown ER, Bone Q (1991) Repetitive stimulation of the squid giant axon exerts graded control over mantle tension. J Mar Biol Assoc UK 71:732
Brown ER, Piscopo S, De Stefano R, Giuditta A (2006) Brain and behavioural evidence for rest-activity cycles in Octopus vulgaris. Behav Brain Res 172:355–359
Brown ER, Piscopo S, Chun J-T, Francone M, Mirabile I, D’Aniello A (2007) Modulation of an AMPA-like glutamate receptor (SqGluR) gating by l- and d-aspartic acids. Amino Acids 32(1):53–57
Budelmann BU, Bullock TH, Williamson R (1995) Cephalopod brains: promising preparations for brain physiology. In: Abbott NJ, Williamson R, Maddock L (eds) Cephalopod neurobiology: neuroscience studies in squid, octopus and cuttlefish. Oxford University Press, London, pp 399–413
Bullock TH (1948) Properties of a single synapse in the stellate ganglion of squid. J Neurophysiol 11:343–364
Bullock TH (1984) Ongoing compound field potentials from octopus brain are labile and vertebrate-like. Electroencephalogr Clin Neurophysiol 57:473–483
Bullock TH, Basar E (1988) Comparison of ongoing compound field potentials in the brains of invertebrates and vertebrates. Brain Res Rev 13:57–75
Charlton MP, Bittner GD (1978a) Facilitation of transmitter release at squid synapses. J Gen Physiol 72:471–486
Charlton MP, Bittner GD (1978b) Presynaptic potentials and facilitation of transmitter release in the squid giant synapse. J Gen Physiol 72:487–511
Clay JR (1986) Potassium ion accumulation slows the closing rate of potassium channels in squid axons. Biophys J 50:197–200
Crispo E (2007) The Baldwin effect and genetic assimilation: revisiting two mechanisms of evolutionary change mediated by phenotypic plasticity. Evolution 61:2469–2479
Cuttle MF, Tsujimoto T, Forsythe ID, Takahashi T (1998) Facilitation of the presynaptic calcium current at an auditory synapse in rat brainstem. J Physiol 512:723–729
Del Castillo J, Katz B (1953) Statistical aspects of transmission at a single nerve muscle junction. J Physiol 120:32
Di Cosmo A, Paolucci M, Di Cristo C (2004) N-methyl-d-aspartate receptor-like immunoreactivity in the brain of Sepia and Octopus. J Comp Neurol 477:202–219
Di Cosmo A, Di Cristo C, Messenger J (2006) l-Glutamate and its ionotropic receptors in the nervous system of cephalopods. Curr Neuropharmacol 4:305–312
Erulkar SD, Weight FF (1977) Extracellular potassium and transmitter release at the giant synapse of squid. J Physiol 266:209–218
Eyman M, Cefaliello C, Ferrara E, De Stefano R, Lavina ZS, Crispino M, Squillace A, van Minnen J, Kaplan BB, Giuditta A (2007) Local synthesis of axonal and presynaptic RNA in squid model systems. Eur J Neurosci 25:341–350
Florey E, Kriebel M (1969) Electrical and mechanical responses of chromatophore muscle fibers of squid, Loligo opalescens, to nerve stimulation and drugs. Z Vgl Physiol 65:98–130
Florey E, Winesdorfer J (1968) Cholinergic nerve endings in Octopus brain. J Neurochem 15:169
Florey E, Dubas F, Hanlon RT (1985) Evidence for l-glutamate as a transmitter substance of motoneurons innervating squid chromatophore muscles. Comp Biochem Physiol C Comp Pharmacol Toxicol 82:259–268
Forsythe ID (1994) Direct patch recording from identified presynaptic terminals mediating glutamatergic EPSCs in the rat CNS, in vitro. J Physiol 479:381–387
Frankenhauser B, Hodgkin AL (1956) The after-effects of impulses in the giant nerve fibres of Loligo. J Physiol 131:341–376
Hanlon RT, Messenger JB (1998) Cephalopod behaviour. Cambridge University Press, Cambridge
Hebb DO (2002) The organization of behavior: a neuropsychological theory. Psychology Press, New York
Hochner B, Brown ER, Langella M, Shomrat T, Fiorito G (2003) A learning and memory area in the octopus brain manifests a vertebrate-like long-term potentiation. J Neurophysiol 90:3547–3554
Hochner B, Shomrat T, Fiorito G (2006) The octopus: a model for a comparative analysis of the evolution of learning and memory mechanisms. Biol Bull 210:308–317
Hodgkin AL, Huxley AF (1952) A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol 117:500–544
Inoue I, Tsutsui I, Brown ER (1997) K+ accumulation and K+ conductance inactivation during action potential trains in giant axons of the squid Sepioteuthis. J Physiol 500:355–366
Kandel ER (1985) Early experience, critical periods and developmental fine tuning of brain architecture. In: Kandel ER, Schwartz JH (eds) Principles of neural science. Elsevier, New York, pp 757–770
Katz B, Miledi R (1966) Input–output relation of a single synapse. Nature 212:1242–1245
Katz B, Miledi R (1968) The role of calcium in neuromuscular facilitation. J Physiol 195:481–492
Kemenes I, Straub VA, Nikitin ES, Staras K, O’Shea M, Kemenes G, Benjamin PR (2006) Role of delayed nonsynaptic neuronal plasticity in long-term associative memory. Curr Biol 16:1269–1279
Konorski J (1948) Conditioned reflexes and neuron organization. Cambridge University Press, New York
Langella M (2005) Mechanisms of synaptic plasticity in the vertical lobe of Octopus vulgaris. PhD. The Open University, UK
Laverack MS (1980) Electrophysiology of the isolated central nervous system of the northern octopus Eledone cirrhosa. Mar Behav Physiol 7:155–169
Lima (2002) Neuronal and local control of squid chromatophore muscle (BL). Ph.D. 2001, Sheffield
Lima PA, Nardi G, Brown ER (2003) AMPA/kainate and NMDA-like glutamate receptors at the chromatophore neuromuscular junction of the squid: role in synaptic transmission and skin patterning. Eur J Neurosci 17:507–516
Loi PK, Saunders RG, Young DC, Tublitz NJ (1996) Peptidergic regulation of chromatophore function in the European cuttlefish Sepia officinalis. J Exp Biol 199:1177–1187
Martin AR, Pilar G (1963) Dual mode of synaptic transmission in the avian ciliary ganglion. J Physiol 168(2):443–463
Mattiello T, Fiore G, Brown ER, d’ Ischia M, Palumbo A (2010) Nitric oxide mediates the glutamate-dependent pathway for neurotransmission in Sepia officinalis chromatophore organs. J Biol Chem 285:24154–24163
Matzner H, Gutfreund Y, Hochner B (2000) Neuromuscular system of the flexible arm of the Octopus: physiological characterization. J Neurophysiol 83:1315–1328
Messenger JB (2001) Cephalopod chromatophores: neurobiology and natural history. Biol Rev 76:473–528
Morris RG, Anderson E, Lynch GS, Baudry M (1986) Selective impairment of learning and blockade of long-term potentiation by an N-methyl-d-aspartate receptor antagonist, AP5. Nature 319:774–776
Nixon M, Young JZ (2003) The brains and lives of cephalopods. Oxford University Press, Oxford
Otis T, Gilly W (1990) Jet-propelled escape in the squid Loligo-opalescens—concerted control. Proc Natl Acad Sci USA 87:2911–2915
Packard A (1972) Cephalopods and fish; the limits of convergence. Biol Rev 47:241–307
Packard A (2006) Contribution to the whole (H). Can squids show us anything that we did not know already? Biol Philos 21:189–211
Piscopo S, Moccia F, Di Cristo C, Caputi L, Di Cosmo A, Brown ER (2007) Pre- and postsynaptic excitation and inhibition at octopus optic lobe photoreceptor terminals; implications for the function of the “presynaptic bags”. Eur J Neurosci 26:2196–2203
Preuss T, Gilly WF (2000) Role of prey- capture experience in the development of the escape response in the squid Loligo opalescens: a physiological correlate in an identified neuron. J Exp Biol 203:559–565
Prosser CL, Young JZ (1937) Responses of muscles of the squid to repetitive stimulation of the giant nerve fibers. Biol Bull 73:237–241
Rahamimoff R (1968) A dual effect of calcium ions on neuromuscular facilitation. J Physiol 195:471–480
Sereni E, Young JZ (1932) Nervous degeneration and regeneration in cephalopods. Pubbl Dell Stn Zool Di Napoli 12(5):176–208
Shomrat T, Graindorge N, Bellanger C, Fiorito G, Loewenstein Y, Hochner B (2011) Alternative sites of synaptic plasticity in two homologous “fan-out fan-in” learning and memory networks. Curr Biol 21:1773–1782
Stanley EF (1989) Calcium currents in a vertebrate presynaptic nerve-terminal: the chick ciliary ganglion calyx. Brain Res 505(2):341–345
Sumbre G, Fiorito G, Flash T, Hochner B (2005) Motor control of flexible octopus arms. Nature 433:595–596
Takeuchi A, Takeuchi N (1962) Electrical changes in pre- and postsynaptic axons of the giant synapse of Loligo. J Gen Physiol 45:1181–1193
Vinogradova I, Zajicek J, Brown ER (1999) Postsynaptic spike generation at the squid giant synapse depends on a calcium-activated conductance. Soc Neurosci Abstr 25:1739
Vinogradova I, Zajicek J, Gentile S, Brown E (2002) Effect of glycine on synaptic transmission at the third order giant synapse of the squids Alloteuthis subulata and Loligo vulgaris. Neurosci Lett 325:42–46
Waddington CH (1942) Canalization of development and the inheritance of acquired characters. Nature 150:563–565
Weight FF, Erulkar SD (1976) Modulation of synaptic transmitter release by repetitive postsynaptic action potentials. Science 193:1023–1025
Wells MJ (1978) Octopus: physiology and behaviour of an advanced invertebrate. Chapman and Hall, London
Williams LW (1910) The anatomy of the common squid Loligo pealii: Lesueur. E J Brill, Leiden
Williamson R, Budelmann BU (1991) Convergent inputs to Octopus oculomotor neurones demonstrated in a brain slice preparation. Neurosci Lett 121:215–218
Williamson R, Ichikawa M, Matsumoto G (1993) Neuronal circuits in cephalopod vision. Neth J Zool 44:272–283
Yawo H (1990) Voltage-activated calcium currents in presynaptic nerve terminals of the chicken ciliary ganglion. J Physiol 428(1):199–213
Young JZ (1935) Structure of nerve fibres in Sepia. J Physiol 83:27P
Young JZ (1963) The number and sizes of nerve cells in Octopus. Proc Zool Soc Lond 140:229–254
Young JZ (1971) The anatomy of the central nervous system of Octopus vulgaris. Claredon Press, Oxford
Young JZ (1991) Computation in the learning system of cephalopods. Biol Bull 180:200–208
Acknowledgments
The authors would like to thank the two anonymous referees whose comments helped to improve the manuscript and Mr G. Ferrandino for help with the artwork.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article forms part of a special issue on Cephalopod Biology under the auspices of CephRes-ONLUS (http://www.cephalopodresearch.org).
Guest Editor: Graziano Fiorito.
Rights and permissions
About this article
Cite this article
Brown, E.R., Piscopo, S. Synaptic plasticity in cephalopods; more than just learning and memory?. Invert Neurosci 13, 35–44 (2013). https://doi.org/10.1007/s10158-013-0150-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10158-013-0150-4