Abstract
Background
The aim of the present study was to estimate the population pharmacokinetic parameters of mizoribine in adult recipients of renal transplantation using a nonlinear mixed effects model (NONMEM) program.
Methods
Pharmacokinetic data for population analysis were retrospectively collected from 114 recipients (66 males and 48 females) routinely treated with oral administration of mizoribine (25–450 mg/day). The range of creatinine clearance (CLcr) was 7.6−136.1 mL/min (mean 49.2 mL/min).
Results
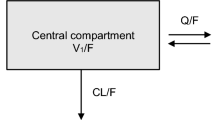
The pharmacokinetics of mizoribine in adult recipients of renal transplantation was well described by a 1-compartment model with first-order absorption. The mean value of the absorption lag time (ALAG) and absorption rate constant (KA) was estimated to be 0.581 and 0.983 h−1, respectively. Apparent volume of distribution (V/F) was modeled as a function of body weight (WT), and the mean value was estimated to be 0.858 × WT L. Oral clearance (CL/F) was modeled as a function of creatinine clearance (CLcr), and the mean value was estimated to be 1.80 × CLcr × 60/1000 L/h. In addition, there was a strong correlation between CLcr-corrected CL/F and WT-corrected V/F in the adult recipients, indicating large interindividual variability in bioavailability (F) of mizoribine.
Conclusion
The present findings suggested that not only the rate of renal excretion but also the extent of intestinal absorption of mizoribine is responsible for the large interindividual pharmacokinetic variability of the drug.




Similar content being viewed by others
References
Yokota S. Mizoribine: mode of action and effects in clinical use. Pediatr Int. 2002;44:196–8.
Morino T, Sano K, Hara H, Motoyama K, Iizuka K, Hara T, et al. A comparative, subacute toxicity study of mizoribine and azathioprine in dogs—with particular reference to hepatotoxicity, nephrotoxicity and hematotoxicity. Jpn J Transplant. 1982;17 Suppl:603–14.
Ihara H, Shinkuma D, Kubo M, Miyamoto I, Nojima M, Koike H, et al. Influence of bioavailability on blood level of mizoribine in kidney transplant recipients. Transplant Proc. 1996;1:1321–3.
Takada K, Asada S, Ichikawa Y, Sonoda T, Takahara S, Nagano S, et al. Pharmacokinetics of bredinin in renal transplant patients. Eur J Clin Pharmacol. 1983;24:457–61.
Honda M, Itoh H, Suzuki T, Hashimoto Y. Population pharmacokinetics of higher-dose mizoribine in healthy male volunteers. Biol Pharm Bull. 2006;29:2460–4.
Beal SL, Boeckmann AJ, Sheiner LB. NONMEM Users Guides: NONMEM Project Group: University of California, San Francisco; 1992.
Ishida K, Takaai M, Yotsutani A, Taguchi M, Hashimoto Y. Membrane transport mechanisms of mizoribine in the rat intestine and human epithelial LS180 cells. Biol Pharm Bull. 2009;32:741–5.
Ishida K, Takaai M, Yotsutani A, Yokota A, Sakamoto T, Taguchi M, et al. Mechanisms responsible for different rates of uptake of mizoribine and ribavirin by human epithelial LS180 cells. Jpn J Pharm Health Care Sci. 2010;36:900–5.
Hosotsubo H, Takahara S, Taenaka N. Simplified high-performance liquid chromatographic method for determination of mizoribine in human serum. J Chromatogr. 1988;432:340–5.
Govindarajan R, Bakken AH, Hudkins KL, Lai Y, Casado FJ, Pastor-Anglada M, et al. In situ hybridization and immunolocalization of concentrative and equilibrative nucleoside transporters in the human intestine, liver, kidneys, and placenta. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1809–22.
Gray JH, Owen RP, Giacomini KM. The concentrative nucleoside transporter family, SLC28. Pflugers Arch. 2004;447:728–34.
Podgorska M, Kocbuch K, Pawelczyk T. Recent advances in studies on biochemical and structural properties of equilibrative and concentrative nucleoside transporters. Acta Biochim Pol. 2005;52:749–58.
Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447:735–43.
Naito T, Tokashiki S, Mino Y, Otsuka A, Ozono S, Kagawa Y, et al. Impact of concentrative nucleoside transporter 1 gene polymorphism on oral bioavailability of mizoribine in stable kidney transplant recipients. Basic Clin Pharmacol Toxicol. 2010;106:310–6.
Sugitani A, Kitada H, Ota M, Yoshida J, Doi A, Hirakata H, et al. Revival of effective and safe high-dose mizoribine for the kidney transplantation. Clin Transplant. 2005;20:590–5.
Sonda K, Takahashi K, Tanabe K, Funchinoue S, Hayasaka Y, Kawaguchi H, et al. Clinical pharmacokinetic study of mizoribine in renal transplantation patients. Transplant Proc. 1996;28:3643–8.
Acknowledgments
The present study was executed as themes of secretaries of the study group of mizoribine on renal transplantation. The data in this study was collected by following 11 hospitals: Kyoto Prefectural University of Medicine, Osaka University, Hyogo College of Medicine, Ihara Clinic, Nara Medical University, Osaka City University, Sakai Hospital Kinki University faculty of Medicine, Kinki University Hospital, Hyogo Prefectural Nishinomiya Hospital, Yamaguchi-ken Saiseikai Shimonoseki General Hospital, and Hiroshima Prefectural Hospital. We are grateful to the doctors in these hospitals.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ishida, K., Okamoto, M., Ishibashi, M. et al. Population pharmacokinetics of mizoribine in adult recipients of renal transplantation. Clin Exp Nephrol 15, 900–906 (2011). https://doi.org/10.1007/s10157-011-0487-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-011-0487-0