Abstract
Influenza infection tends to be severe in patients with chronic underlying diseases. This study evaluated the efficacy and safety of laninamivir octanoate, an inhaled neuraminidase inhibitor, for the treatment of influenza patients with chronic respiratory diseases; we conducted a double-blind, randomized controlled trial to compare the efficacy and safety of laninamivir octanoate and oseltamivir for the treatment of influenza in these patients. A total of 203 patients aged ≥20 years were randomized to receive either laninamivir octanoate or oseltamivir. The primary efficacy endpoint was the time to illness alleviation. This study is registered with JapicCTI; the registration number is JapicCTI-090940. The full analysis set (FAS) included a total of 201 patients (laninamivir group, n = 101; oseltamivir group, n = 100). Most patients had underlying bronchial asthma and 170 patients were infected with influenza A(H1N1)2009. The median time to illness alleviation was 64.7 h in the laninamivir group and 59.7 h in the oseltamivir group, with a difference of 5.0 h between the two groups (95 % confidence interval, −13.6 to 16.1 h). No adverse events specific to laninamivir octanoate were observed, and adverse events such as bronchospasm, which has been reported to be observed with other inhaled drugs related to laninamivir octanoate, did not occur. Laninamivir octanoate showed similar efficacy and safety to oseltamivir in the treatment of influenza, including that caused by influenza A(H1N1)2009, in patients with chronic respiratory diseases.
Similar content being viewed by others
Introduction
Influenza is a seasonal respiratory infection, and can be associated with serious illness, or even death, particularly in high-risk populations, such as the elderly, children, pregnant women, and patients with underlying respiratory and cardiac diseases, raising medical and social concerns [1]. In recent years, the influenza pandemic caused by the highly pathogenic H5N1 avian influenza virus has been a concern worldwide, but a pandemic of influenza A(H1N1)2009 occurred in 2009. It has been suggested that influenza A(H1N1)2009 infection also tends to cause serious illness in high-risk patients [2], and the necessity for early treatment with neuraminidase inhibitors has been increasingly clearly recognized [3–6]. On the other hand, the worldwide spread of oseltamivir-resistant H1N1 virus carrying the H274Y mutation has also been of great concern [7–9]. Oseltamivir resistance has also been reported in influenza A(H1N1)2009, although at a low frequency [10].
Laninamivir octanoate, a prodrug of laninamivir, is a long-acting neuraminidase inhibitor which exerts its beneficial effect by remaining in the target organs; namely, the trachea and lung, for a long time. It has been confirmed that a single inhalation of laninamivir octanoate has an effect not inferior to that of the existing neuraminidase inhibitors against influenza A and B infection [11, 12]. In addition, a non-clinical study has confirmed that laninamivir exhibits neuraminidase inhibitory activity against influenza A and B viruses, including the highly pathogenic H5N1, oseltamivir-resistant viruses [13] and influenza A(H1N1)2009 [14]. From the above findings, laninamivir octanoate is considered to be a potentially useful drug that would expand the spectrum of drug choices for the treatment of influenza infection. In September 2010, laninamivir octanoate was approved for manufacturing and marketing in Japan.
However, the efficacy and safety of laninamivir octanoate have not yet been sufficiently evaluated for the treatment of influenza infection in patients with underlying chronic respiratory diseases. It has been reported that influenza A(H1N1)2009 infection may cause serious illness, particularly in high-risk patients such as those with chronic respiratory diseases [2]. In addition, zanamivir, an inhaled drug related to laninamivir octanoate, has been reported to cause bronchospasm and decreased respiratory function [15, 16].
Against this background, we conducted a double-blind, randomized controlled trial, using oseltamivir (which is the most widely used anti-influenza agent) as the control drug, to investigate the efficacy and safety of laninamivir octanoate for the treatment of influenza in patients with underlying chronic respiratory diseases.
Patients and methods
Study design and patients
This multicenter, double-blind, randomized controlled trial was conducted from November 2009 through March 2011 at 53 facilities in Japan. This study was undertaken in accordance with the Declaration of Helsinki and Good Clinical Practice (GCP) guidelines, and the study protocol was approved by the appropriate ethics committee at each institution. In addition, written informed consent was obtained from all patients. This study is registered with JapicCTI; the registration number is JapicCTI-090940.
The inclusion criteria were patients aged ≥20 years, having underlying chronic respiratory diseases, within 36 h of the onset of influenza symptoms, an axillary temperature of ≥37.5 °C, and diagnosis of influenza infection by the investigator. The exclusion criteria were as follows: suspected infection with bacteria or non-influenza viruses within 1 week prior to provision of consent; reported occurrence of any influenza-like symptom that cannot be distinguished from those of underlying diseases within 1 week prior to provision of consent; continuous use of steroids at a prednisolone-equivalent dose of more than 10 mg prior to provision of consent; bronchial asthma attack of at least moderate severity at the time of provision of consent; chronic respiratory failure; renal dysfunction; history of alcohol or drug abuse; and treatment with amantadine or other neuraminidase inhibitors within 4 weeks. Pregnant women, breastfeeding women, and women who wished to become pregnant during the study period were also excluded.
In addition, patients were allowed to use acetaminophen as a rescue medication for influenza symptom relief, and its use was recorded.
Randomization and masking
Patients were randomly assigned (1:1) to either the laninamivir group or the oseltamivir group. Patients in the laninamivir group were administered 40 mg of laninamivir octanoate as a single inhalation on day 1 and oseltamivir phosphate placebo capsules 2 times per day for 5 days. Patients in the oseltamivir group were administered one capsule (75 mg) of oseltamivir phosphate orally twice daily for 5 days (days 1–5) and laninamivir octanoate placebo powder once on day 1. A computer-generated block random allocation sequence was provided by ACRONET Corporation (Tokyo, Japan) and was stratified according to the institution and type of influenza virus on the basis of the results of a rapid diagnosis kit capable of separately detecting influenza A and B. The patients, investigators, and trial personnel were blinded to the allocation sequence throughout the trial with use of a double-dummy method. The initial administration of the test drugs was confirmed by an investigator.
Procedures
Medical histories, complications, vital signs, physical examinations, and baseline virological samples were obtained before treatment on day 1. Hematology, blood chemistry, and urinalysis were performed on day 1 (baseline) and day 15 for the safety assessment. Patients recorded their axillary temperature and severity of influenza symptoms (headache, myalgia/arthralgia, fatigue, chills/sweats, nasal symptoms, sore throat, and cough) 4 times daily for 15 days. The severity of each influenza symptom was graded into 4 categories (0, absent; 1, mild; 2, moderate; 3, severe) and was measured as the symptom score.
Influenza infection was confirmed by laboratory virological tests. Anterior nose and/or posterior pharyngeal throat swabs were taken on days 1 (baseline), 3, and 6 (±1 day for days 3 and 6) and were placed in a viral transport medium. The swab samples were eluted and frozen at −80 ± 10 °C until use. For the baseline assessment, the subtype of influenza (seasonal H1N1, H3N2, and B) was determined based on the amplified DNA size by a reverse-transcription polymerase chain reaction (RT-PCR) with subtype-specific primers designed from the hemagglutinin sequences of seasonal H1N1, H3N2, and B viruses. Influenza A(H1N1)2009 was determined by a real-time RT-PCR. Susceptibilities to laninamivir and oseltamivir carboxylate were determined by a fluorescence-based neuraminidase inhibition assay using culture supernatants propagated once from the thawed swab samples in Madin–Darby canine kidney (MDCK) cells. The detection of mutations was confirmed by analyzing gene sequences of viruses in which drug resistance was suspected based on the results of the neuraminidase inhibition assay. Virus titers were determined using the swab samples obtained at 3 time points. The thawed swab samples were serially diluted and cultured for 7 days in MDCK cells. Based on the dilution factor showing no cytopathic effect, the virus titers were calculated as the log10 50 % tissue culture infective dose (TCID50) per mL of the viral transport medium, according to the Behrens–Kärber equation [17]. Serum samples were obtained on days 1 and 15 (−2 to +7 days) and were used to perform a hemagglutination-inhibition assay. The serological response was defined as a 4-fold or greater increase in type- or subtype-specific antibody on day 15, as compared with that on day 1. If the sample results were negative by RT-PCR, real-time RT-PCR, and the hemagglutination-inhibition assay, the patient was regarded as not having a laboratory-confirmed influenza virus infection. All the virological tests were performed at Mitsubishi Chemical Medience (Tokyo, Japan).
Study outcomes
The primary endpoint was the time to illness alleviation, defined as the time from the initiation of the trial treatment to the beginning of the first 21.5-h period in which all influenza symptoms were “absent” or “mild”. The time to illness alleviation was defined as reported in previous clinical trials of laninamivir and oseltamivir [11, 18–21]. Patients whose influenza symptoms had not resolved by the time of their withdrawal from the study or by the end of the observation period were censored. In addition, the incidence of influenza-associated complications and the rates of exacerbation of the underlying disease were also calculated for each treatment group.
Statistical analysis
The target number of patients was 100 for each group, from the standpoint of efficacy and safety. The median time to illness alleviation in high-risk patients treated with oseltamivir phosphate was estimated from previous studies to be 150 h. The target number of patients was established using Monte Carlo simulation so that the probability that the difference in the median time to illness alleviation was <24 h was 70–80 % on the assumption that the effect would be similar in the laninamivir and oseltamivir groups. Adverse events with an incidence of 3 % could be detected with a probability of 95 % or more in a group of 100 patients.
In the efficacy analysis, the difference in the median time to illness alleviation between the laninamivir and oseltamivir groups was calculated, and its 2-sided nonparametric 95 % confidence interval on the basis of the generalized Wilcoxon test was calculated. In addition, a generalized Wilcoxon’s test was performed using the oseltamivir group as the control. Furthermore, the proportion of patients who developed influenza-associated complications; namely, pneumonia, bronchitis, otitis media, and sinusitis, and the proportion of patients in whom the underlying disease was exacerbated after the start of treatment were calculated for each treatment group. All analyses were performed using the SAS System, Release 8.2 (SAS Institute, Cary, NC, USA). All reported P values were 2-sided, without adjustments for multiple testing.
In the efficacy analysis, the full analysis set (FAS) [22] based on the intention-to-treat principle was defined as the primary analysis set, and the per protocol set (PPS) [22] was used for the sensitivity analysis. The safety analysis included all patients who had received at least 1 dose of the trial treatment, and had undergone at least 1 safety assessment.
Results
Details of the subjects are shown in Fig. 1. Informed consent was obtained from 204 influenza-infected patients with chronic respiratory diseases, and 203 patients were randomized. Of these, 1 patient assigned to the oseltamivir group was excluded from all the analyses because of discontinuation from the trial before receiving any treatment. And 1 patient assigned to the laninamivir group was excluded from the FAS, because this patient had no influenza symptom data during the study period. From the above, a total of 201 patients were included in the FAS (laninamivir group, n = 101; oseltamivir group, n = 100).
The baseline characteristics of the patients were well balanced between the 2 groups in both the FAS (Table 1) and the PPS (data not shown). Most patients had underlying bronchial asthma. In addition, approximately 90 % of the patients in whom the virus type and subtype could be identified by laboratory virological tests were infected with influenza A(H1N1)2009, and the remaining patients were infected with H3N2. None of the patients were infected with the seasonal H1N1 or B. The H274Y mutation was found in 3 patients in whom influenza A(H1N1)2009 was isolated on day 1 (baseline).
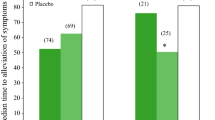
The time courses for illness alleviation were almost the same in the 2 groups (Fig. 2). In the FAS, the median time to illness alleviation was 64.7 h in the laninamivir group and 59.7 h in the oseltamivir group, with a difference of 5.0 h between the two groups (95 % CI −13.6 to 16.1 h, P = 0.881; Table 2). The median time to alleviation of influenza A(H1N1)2009 was also similar in the laninamivir and oseltamivir groups. The median time to return to normal axillary temperature was 48.9 h in the laninamivir group and 43.6 h in the oseltamivir group. Similar results were also obtained in the PPS (Table 2).
There was no significant difference between the 2 groups in the median time to alleviation of the influenza symptoms (headache, myalgia/arthralgia, fatigue, chills/sweats, nasal symptoms, sore throat, and cough) after the start of treatment. However, all symptoms, except for the nasal symptoms, tended to be alleviated more rapidly in the laninamivir group than in the oseltamivir group (Fig. 3).
Both the incidence of influenza-associated complications (pneumonia, bronchitis, otitis media, and sinusitis) and the rate of exacerbation of the underlying disease were similar in the 2 groups (Table 2).
The proportion of patients shedding virus on day 1 (baseline) was similar in the 2 groups. The proportion of patients shedding virus on day 3 tended to be higher in the laninamivir group (55.7 %) than that in the oseltamivir group (45.4 %), although the difference was not significant (Table 3).
As for safety, both of the drugs were well tolerated. Adverse events that occurred in at least 3 % of the patients in the laninamivir group included asthma (exacerbation of asthma; 5.9 %, 6/102), diarrhea (3.9 %, 4/102), and bronchitis (3.9 %, 4/102). These adverse events were observed at similar frequencies in the oseltamivir group, with the corresponding event rates being 7 % (7/100), 5 % (5/100), and 3 % (3/100), respectively. All of these adverse events were mild to moderate in severity and resolved or improved with drug or other treatments. As the only serious adverse event, visual hallucination developed in 1 patient in the oseltamivir group.
Discussion
The median time to illness alleviation, which was the primary efficacy endpoint in this study, was similar in the laninamivir (64.7 h) and oseltamivir (59.7 h) groups. In addition, the median time to alleviation of influenza A(H1N1)2009 was also similar in the 2 groups. In a phase III trial conducted in the 2008–2009 influenza season, the median time to illness alleviation was 73.0 h in the laninamivir group and 73.6 h in the oseltamivir group [11], and in both groups, recovery from influenza was advanced by about 10 h in the present study as compared with that in the above phase III trial. The present study was conducted after a pandemic, and approximately 90 % of the patients diagnosed to have influenza were infected with influenza A(H1N1)2009. The pathogenicity of influenza A(H1N1)2009 has been reported to be higher than that of the seasonal H1N1 [14, 23]. On the other hand, it has also been reported that the mean time to return to normal temperature is shorter in infections caused by A(H1N1)2009 than in those caused by the seasonal H1N1 [24]. From the results of the present study, it was speculated that the clinical symptoms of influenza A(H1N1)2009 infection may be milder than those of influenza caused by the seasonal H1N1.
Chronic respiratory disease is known to be a high-risk factor for the development of complications in patients with influenza infection, and such disease is also known to be associated with a high risk of serious illness caused by secondary bacterial infection and exacerbation of the underlying disease in these patients. A comparison between our laninamivir and oseltamivir groups revealed that the incidence of influenza-associated complications and the rates of exacerbation of the underlying disease were similar in the 2 groups. It has been reported that oseltamivir decreases the risk of secondary infection and hospitalization associated with influenza virus infection [25, 26], suggesting that laninamivir octanoate may similarly decrease the risk of development of serious illness/complications associated with influenza.
The mean time from the onset of influenza to the end of treatment was 22.86 h in our laninamivir group and 22.60 h in the oseltamivir group. It can be speculated that patients may have recovered without developing more severe symptoms because of the early treatment with the neuraminidase inhibitors.
In this study, most patients had relatively mild bronchial asthma, and patients with poorly controlled or severe bronchial asthma were not included. Only a few patients with chronic respiratory diseases other than bronchial asthma were included. Therefore, in this study, the efficacy and safety of laninamivir octanoate in patients with underlying chronic respiratory diseases other than mild bronchial asthma could not be sufficiently investigated. However, cough and fatigue, which are also symptoms of patients with chronic respiratory diseases, tended to resolve more quickly in the laninamivir group than in the oseltamivir group, suggesting that laninamivir octanoate may also be a useful drug from the standpoint of controlling the underlying diseases.
As for safety, both of the drugs were well tolerated. No adverse events specific to laninamivir octanoate were observed, and adverse events suggesting decreased respiratory function, such as bronchospasm, which has been reported to be observed with other inhaled drugs related to laninamivir octanoate, did not occur.
It was confirmed in the present study that a single inhalation of laninamivir octanoate can also be used safely for the treatment of influenza in patients with chronic respiratory diseases, such as bronchial asthma, and that laninamivir octanoate exhibits similar efficacy to oseltamivir. In addition, laninamivir octanoate also showed a similar effect to oseltamivir against influenza A(H1N1)2009 infection.
References
Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, et al. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2008. MMWR Recomm Rep. 2008;57:1–60.
The City of New York Department of Health and Mental Hygiene. In: New York City Department of Health and Mental Hygiene Health Alert #22: Novel H1N1 Influenza Update June 12, 2009. 2009. http://www.nyc.gov/html/doh/downloads/pdf/cd/2009/09md22.pdf. Accessed 12 March 2012.
Zarychanski R, Stuart TL, Kumar A, Doucette S, Elliott L, Kettner J, et al. Correlates of severe disease in patients with 2009 pandemic influenza (H1N1) virus infection. CMAJ. 2010;182:257–64.
Kumar A. Early versus late oseltamivir treatment in severely ill patients with 2009 pandemic influenza A (H1N1): speed is life. J Antimicrob Chemother. 2011;66:959–63.
Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–44.
Fiore AE, Fry A, Shay D, Gubareva L, Bresee JS, Uyeki TM. Antiviral agents for the treatment and chemoprophylaxis of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1–24.
World Health Organization. Influenza A (H1N1) virus resistance to oseltamivir—2008/2009 influenza season, northern hemisphere. http://www.who.int/influenza/resources/documents/H1N1webupdate20090318_ed_ns.pdf. Accessed 12 March 2012.
Kawai N, Ikematsu H, Iwaki N, Kondou K, Hirotsu N, Kawashima T, et al. Clinical effectiveness of oseltamivir for influenza A (H1N1) virus with H274Y neuraminidase mutation. J Infect. 2009;59:207–12.
Centers for Disease Control and Prevention. Oseltamivir-resistant novel influenza A (H1N1) virus infection in two immunosuppressed patients—Seattle, Washington, 2009. MMWR Morb Mortal Wkly Rep. 2009;58:893–6.
Graitcer SB, Gubareva L, Kamimoto L, Doshi S, Vandermeer M, Louie J, et al. Characteristics of patients with oseltamivir-resistant pandemic (H1N1) 2009—United States. Emerg Infect Dis. 2011;17:255–7.
Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis. 2010;51:1167–75.
Sugaya N, Ohashi Y. Long-acting neuraminidase inhibitor laninamivir octanoate (CS-8958) versus oseltamivir as treatment for children with influenza virus infection. Antimicrob Agents Chemother. 2010;54:2575–82.
Yamashita M, Tomozawa T, Kakuta M, Tokumitsu A, Nasu H, Kubo S. CS-8958, a prodrug of the new neuraminidase inhibitor R-125489, shows long-acting anti-influenza virus activity. Antimicrob Agents Chemother. 2009;53:186–92.
Itoh Y, Shinya K, Kiso M, Watanabe T, Sakoda Y, Hatta M, et al. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature. 2009;460:1021–5.
Williamson JC, Pegram PS. Respiratory distress associated with zanamivir. N Engl J Med. 2000;342:661–2.
RelenzaTM: Summary of product characteristics. Brentford: GlaxoSmithKline; 2011.
Behrens B, Kärber G. Wie sind Rehenversuche für biologishe Auswertungen am zweckmäsigsten anzuordnen? (in German). Naunyn Schmiedebergs Arch Pharmakol Exp Pathol. 1935;177:379–88.
Cooper NJ, Sutton AJ, Abrams KR, Wailoo A, Turner D, Nicholson KG. Effectiveness of neuraminidase inhibitors in treatment and prevention of influenza A and B: systematic review and meta-analyses of randomised controlled trials. BMJ. 2003;326:1235–40.
Treanor JJ, Hayden FG, Vrooman PS, Barbarash R, Bettis R, Riff D, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: a randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000;283:1016–24.
Nicholson KG, Aoki FY, Osterhaus AD, Trottier S, Carewicz O, Mercier CH, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000;355:1845–50 (Erratum, Lancet 2000;356:1856).
Kashiwagi S, Kudoh S, Watanabe A, Yoshimura I. Clinical efficacy and safety of the selective oral neuraminidase inhibitor oseltamivir in treating acute influenza—placebo-controlled double-blind multicenter phase III trial (in Japanese). Kansenshogaku Zasshi. 2000;74:1044–61.
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonized Tripartite Guideline: Statistical principles for clinical trials E9. 1998. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E9/Step4/E9_Guideline.pdf. Accessed 12 March 2012.
van den Brand JM, Stittelaar KJ, van Amerongen G, Rimmelzwaan GF, Simon J, de Wit E, et al. Severity of pneumonia due to new H1N1 influenza virus in ferrets is intermediate between that due to seasonal H1N1 virus and highly pathogenic avian influenza H5N1 virus. J Infect Dis. 2010;201:993–9.
Kawai N, Hirotsu N, Ikematsu H. Symptoms and complications of influenza. In: The Influenza Study Group of Japan Physicians Association, eds. Clinical practice manual for influenza. 5th edn. Tokyo: Japan Physicians Association; 2010. p. 4–6 (in Japanese).
McCullers JA, Bartmess KC. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J Infect Dis. 2003;187:1000–9.
Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med. 2003;163:1667–72.
Acknowledgment
This work was supported by Daiichi Sankyo Co., Ltd.
Conflict of interest
A. Watanabe has received consultation fees from Daiichi Sankyo Co., Ltd.; Mitsubishi Tanabe Pharma Corporation; Toyama Chemical Co., Ltd.; Otsuka Pharmaceutical Co., Ltd.; GlaxoSmithKline K.K.; and Mitsubishi Chemical Medience.
Author information
Authors and Affiliations
Corresponding author
Appendix: Investigators who enrolled at least 1 patient
Appendix: Investigators who enrolled at least 1 patient
Yoshitaka Sugawara, Hironi Makita, Kouki Kikuchi, Makoto Koizumi, Hideki Nakazawa, Yasuo Tanno, Takefumi Saito, Shinichi Tomioka, Masahiko Tokushima, Masayuki Iijima, Daisuke Uno, Motohiro Kurosawa, Akihiko Ohwada, Susumu Sakayori, Yasuo To, Yoshitaka Nakamori, Norio Yamaguchi, Kazuhiko Oki, Naruhiko Sugihara, Gen Inoue, Hisaho Takahashi, Takayuki Suzuki, Rokuro Matsuoka, Naganobu Hayashi, Hiroyuki Numata, Yoshinori Hasegawa, Tadayuki Terada, Hideki Yasuda, Hirohide Yoneyama, Hiroki Hara, Isao Tanaka, Susumu Yagi, Osamu Moriya, Shin Kawahara, Takafumi Tsuya, Soichiro Hozawa, Toshiyuki Ishimaru, Hirohisa Ichikawa, Kouichi Kimura, Tohru Tsuda, Masayoshi Abe, Yuji Kawarada, Yoshiaki Utsunomiya, Shigeru Fujii, Shinichi Osaki, Koichi Fukuda, Toyomitsu Sawai, Yoshihiro Yamamoto, Yuichi Fukuda, Kiyoyasu Fukushima, Youichi Karimata, Hiroshi Sakugawa, and Hiroshi Nakamura.
About this article
Cite this article
Watanabe, A. A randomized double-blind controlled study of laninamivir compared with oseltamivir for the treatment of influenza in patients with chronic respiratory diseases. J Infect Chemother 19, 89–97 (2013). https://doi.org/10.1007/s10156-012-0460-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10156-012-0460-1