Abstract
Background
The aims of this study are to evaluate the efficacy and safety of first-line treatment with chemotherapy plus cetuximab in real-world patients with recurrent or metastatic squamous cell carcinoma of the head and neck (RM-SCCHN) and to identify prognostic factors for overall survival (OS).
Methods
This is a prospective observation study involving 20 oncology institutions in Japan. Patients with RM-SCCHN treated with a first-line therapy consisting of cetuximab plus any chemotherapy regimen between December 2013 and February 2017 were enrolled. The primary objective of the study was 1-year OS. Secondary objectives included response rate and adverse events.
Results
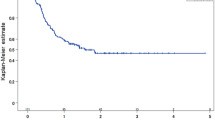
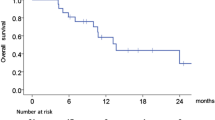
Of 120 patients recruited, 114 patients were analyzed. Median age was 64 years. Cetuximab in combination with platinum plus 5-FU (EXTREME regimen) was chosen in 86 patients (75.4%). The median OS was 12.4 months. A point estimate of the 1-year survival rate was 51.1%. Overall response rate was 26.3%. Grade 3 or worse adverse events included neutropenia (22.8%), hypokalemia (9.6%), acneiform rash (7.0%), pneumonitis (1.8%), and infusion-related reaction (0.9%). On multivariate analysis, regional lymph node metastasis, absence of intervention by dermatologists, lack of response to therapy, skin metastasis, and non-EXTREME regimen were identified as independent unfavorable prognostic factors for OS.
Conclusion
The combination of cetuximab plus chemotherapy was tolerable and efficacious in patients with RM-SCCHN in a real-world setting. Clinical outcomes and prognostic factors extracted from this study provide a reference of the current clinical practice as well as for the future development of novel therapy in RM-SCCHN.

Similar content being viewed by others
References
Baxi S, Fury M, Ganly I et al (2012) Ten years of progress in head and neck cancers. J Natl Compr Canc Netw 10:806–810
Jacobs C, Lyman G, Velez-García E et al (1992) A phase III randomized study comparing cisplatin and fluorouracil as single agents and in combination for advanced squamous cell carcinoma of the head and neck. J Clin Oncol 10:257–263
Forastiere AA, Metch B, Schuller DE et al (1992) Randomized comparison of cisplatin plus fluorouracil and carboplatin plus fluorouracil versus methotrexate in advanced squamous-cell carcinoma of the head and neck. J Clin Oncol 10:1245–1251
Grandis JR, Melhem MF, Gooding WE et al (1998) Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst 90:824–832
Egloff AM, Grandis J (2006) Epidermal growth factor receptor-targeted molecular therapeutics for head and neck squamous cell carcinoma. Expert Opin Ther Targets 10:639–647
Chung CH, Ely K, McGavran L et al (2006) Increased epidermal growth factor receptor gene copy number is associated with poor prognosis in head and neck squamous cell carcinomas. J Clin Oncol 24:4170–4176
Vermorken JB, Mesia R, Rivera F et al (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116–1127
Yoshino T, Hasegawa Y, Takahashi S et al (2013) Platinum-based chemotherapy plus cetuximab for the first-line treatment of Japanese patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: results of a phase II trial. Jpn J Clin Oncol 43:524–531
Burtness B, Harrington KJ, Greil R et al (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394:1915–1928
Ferris RL, Blumenschein G Jr, Fayette J et al (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375:1856–1867
Vermorken JB, Trigo J, Hitt R et al (2007) Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol 25:2171–2177
Hitt R, Irigoyen A, Cortes-Funes H et al (2012) Phase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Ann Oncol 23:1016–1022
Bernad IP, Trufero JM, Urquizu LC et al (2017) Activity of weekly paclitaxel-cetuximab chemotherapy in unselected patients with recurrent/metastatic head and neck squamous cell carcinoma: prognostic factors. Clin Transl Oncol 19:769–776
Knoedler M, Gauler TC, Gruenwald V et al (2013) Phase II study of cetuximab in combination with docetaxel in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck after platinum-containing therapy: a multicenter study of the Arbeitsgemeinschaft Internistische Onkologie. Oncology 84:284–289
Posch D, Fuchs H, Kornek G et al (2016) Docetaxel plus cetuximab biweekly is an active regimen for the first-line treatment of patients with recurrent/metastatic head and neck cancer. Sci Rep 6:32946
Tahara M, Kiyota N, Yokota T et al (2018) Phase II trial of combination treatment with paclitaxel, carboplatin and cetuximab (PCE) as first-line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CSPOR-HN02). Ann Oncol 29:1004–1009
Freemantle N, Strack T (2010) Real-world effectiveness of new medicines should be evaluated by appropriately designed clinical trials. J Clin Epidemiol 63:1053–1058
Berger ML, Lipset C, Gutteridge A et al (2015) Optimizing the leveraging of real-world data to improve the development and use of medicines. Value Health 18:127–130
Depenni R, Cossu Rocca M, Ferrari D et al (2019) Clinical outcomes and prognostic factors in recurrent and/or metastatic head and neck cancer patients treated with chemotherapy plus cetuximab as first-line therapy in a real-world setting. Eur J Cancer 115:4–12
Bossi P, Resteghini C, Paielli N et al (2016) Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget 7:74362–74379
Argiris A, Li Y, Forastiere A (2004) Prognostic factors and long-term survivorship in patients with recurrent or metastatic carcinoma of the head and neck. Cancer 101:2222–2229
Van Cutsem E, Tejpar S, Vanbeckevoort D et al (2012) Intrapatient cetuximab dose escalation in metastatic colorectal cancer according to the grade of early skin reactions: the randomized EVEREST study. J Clin Oncol 30:2861–2868
Bonner JA, Harari PM, Giralt J et al (2010) Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival [published correction appears in Lancet Oncol 2010 Jan; 11(1):14]. Lancet Oncol 11:21–28
Qi WX, Fu S, Zhang Q et al (2014) Incidence and risk of severe infections associated with anti-epidermal growth factor receptor monoclonal antibodies in cancer patients: a systematic review and meta-analysis. BMC Med 12:203
Burtness B, Goldwasser MA, Flood W et al (2005) Eastern Cooperative Oncology Group. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 23:8646–8654
Vermorken JB, Stöhlmacher-Williams J, Davidenko I et al (2013) Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol 14:697–710
Acknowledgements
This study was supported by the Japanese Radiation Oncology Study Group (JROSG) and financially supported by Merck Biopharma. We thank all participating patients and investigators for their support of the study, and gratefully acknowledge the help of all staff at the Clinical Research Institute, National Hospital Organization Kyushu Cancer Center, with study coordination and data collection.
Funding
This work was supported by Merck Biopharma.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Yokota serves in an advisory role for Merck Biopharma, MSD, and Rakuten Medical, and has received lecture fees from Merck Biopharma, Ono Pharmaceutical Co., Ltd., Bristol-Myers Squibb, AstraZeneca, Chugai, MSD, and Eisai. All authors received research funding from Merck Biopharma.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Yokota, T., Ota, Y., Fujii, H. et al. Real-world clinical outcomes and prognostic factors in Japanese patients with recurrent or metastatic squamous cell carcinoma of head and neck treated with chemotherapy plus cetuximab: a prospective observation study (JROSG12-2). Int J Clin Oncol 26, 316–325 (2021). https://doi.org/10.1007/s10147-020-01817-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01817-4