Abstract
Background
The standard of care for first-line treatment of recurrent and/or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN) in patients who cannot tolerate platinum-based regimens has not been clarified. We aimed to develop a new treatment regimen for patients with R/M SCCHN who are ineligible for platinum-based therapy, by evaluating the effects and safety of tegafur/gimeracil/oteracil (S-1) and cetuximab.
Methods
Platinum-ineligibility was defined as: elderly (aged ≥ 75 years), poor PS, comorbidity, platinum resistance and refusal to undergo platinum-based therapy. Patients received S-1 (80 mg/m2/day for 14 days followed by a seven-day break) and cetuximab (initial dose, 400 mg/m2, followed by 250 mg/m2 weekly) until disease progression or unacceptable toxicity. The primary endpoint was overall response rate (ORR).
Results
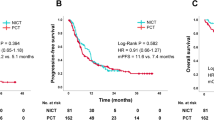
Between September 2014 and September 2018, we enrolled 23 patients. Among the 21 patients who were evaluable, 20 were male [median age, 69 years (range 49–82)]. The ORR was 9 (43%) of 21 patients [95% confidence interval (CI) 22–66]. One and eight patients achieved complete response (CR) and partial response (PR), respectively. The median overall survival (OS) was 13.7 months (95% CI 9.0–18.3) and progression-free survival (PFS) was 5.7 months (95% CI 3.1–8.2). Grade 3/4 adverse events included acneiform rash and skin reactions (33%), hypomagnesemia (19%), hand-foot syndrome (14%), fatigue (14%), mucositis (10%), and anorexia (10%).
Conclusions
Combination treatment with S-1 and cetuximab was effective and tolerated well by patients with platinum-ineligible R/M SCCHN.
Registered clinical trial number: UMIN000015123


Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Pignon JP, le Maitre A, Maillard E et al (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92(1):4–14. https://doi.org/10.1016/j.radonc.2009.04.014
Bernier J, Domenge C, Ozsahin M et al (2004) Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med 350(19):1945–1952. https://doi.org/10.1056/NEJMoa032641
Cooper JS, Pajak TF, Forastiere AA et al (2004) Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med 350(19):1937–1944. https://doi.org/10.1056/NEJMoa032646
Vermorken JB, Specenier P (2010) Optimal treatment for recurrent/metastatic head and neck cancer. Ann Oncol. https://doi.org/10.1093/annonc/mdq453
National Comprehensive Cancer Network Head and Neck Cancers (Version 3.2019) https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck_blocks.pdf. Accessed February 23, 2020
Vermorken JB, Mesia R, Rivera F et al (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359(11):1116–1127. https://doi.org/10.1056/NEJMoa0802656
Shirasaka T, Nakano K, Takechi T et al (1996) Antitumor activity of 1 M tegafur-04 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res 56(11):2602–2606
Sakuramoto S, Sasako M, Yamaguchi T et al (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357(18):1810–1820. https://doi.org/10.1056/NEJMoa072252
Koizumi W, Narahara H, Hara T et al (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9(3):215–221. https://doi.org/10.1016/S1470-2045(08)70035-4
Yamada Y, Takahari D, Matsumoto H et al (2013) Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S-1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol 14(13):1278–1286. https://doi.org/10.1016/S1470-2045(13)70490-X
Muro K, Boku N, Shimada Y et al (2010) Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid plus irinotecan (FOLFIRI) as second-line chemotherapy for metastatic colorectal cancer: a randomised phase 2/3 non-inferiority study (FIRIS study). Lancet Oncol 11(9):853–860. https://doi.org/10.1016/S1470-2045(10)70181-9
Uesaka K, Boku N, Fukutomi A et al (2016) Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 388(10041):248–257. https://doi.org/10.1016/S0140-6736(16)30583-9
Ueno H, Ioka T, Ikeda M et al (2013) Randomized phase III study of gemcitabine plus S-1, S-1 alone, or gemcitabine alone in patients with locally advanced and metastatic pancreatic cancer in Japan and Taiwan: GEST study. J Clin Oncol 31(13):1640–1648. https://doi.org/10.1200/JCO.2012.43.3680
Kubota K, Sakai H, Katakami N et al (2015) A randomized phase III trial of oral S-1 plus cisplatin versus docetaxel plus cisplatin in Japanese patients with advanced non-small-cell lung cancer: TCOG0701 CATS trial. Ann Oncol 26(7):1401–1408. https://doi.org/10.1093/annonc/mdv190
Okamoto I, Yoshioka H, Morita S et al (2010) Phase III trial comparing oral S-1 plus carboplatin with paclitaxel plus carboplatin in chemotherapy-naive patients with advanced non-small-cell lung cancer: results of a west Japan oncology group study. J Clin Oncol 28(36):5240–5246. https://doi.org/10.1200/JCO.2010.31.0326
Takashima T, Mukai H, Hara F et al (2016) Taxanes versus S-1 as the first-line chemotherapy for metastatic breast cancer (SELECT BC): an open-label, non-inferiority, randomised phase 3 trial. Lancet Oncol 17(1):90–98. https://doi.org/10.1016/S1470-2045(15)00411-8
Yokota T, Onozawa Y, Boku N et al (2011) S-1 monotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck after progression on platinum-based chemotherapy. Jpn J Clin Oncol 41(12):1351–1357. https://doi.org/10.1093/jjco/hyr147
Kubota A, Nakatani E, Tsukahara K et al (2018) Adjuvant chemotherapy with S-1 after curative chemoradiotherapy in patients with locoregionally advanced squamous cell carcinoma of the head and neck: reanalysis of the ACTS-HNC study. PLoS ONE 13(6):e0198391. https://doi.org/10.1371/journal.pone.0198391
Sakata K, Someya M, Matsumoto Y et al (2011) Gimeracil, an inhibitor of dihydropyrimidine dehydrogenase, inhibits the early step in homologous recombination. Cancer Sci 102(9):1712–1716. https://doi.org/10.1111/j.1349-7006.2011.02004.x
Tahara M, Araki K, Okano S et al (2011) Phase I trial of combination chemotherapy with docetaxel, cisplatin and S-1 (TPS) in patients with locally advanced or recurrent/metastatic head and neck cancer. Ann Oncol 22(1):175–180. https://doi.org/10.1093/annonc/mdq298
Tahara M, Kiyota N, Mizusawa J et al (2015) Phase II trial of chemoradiotherapy with S-1 plus cisplatin for unresectable locally advanced head and neck cancer (JCOG0706). Cancer Sci 106(6):726–733. https://doi.org/10.1111/cas.12657
Fukuda K, Saikawa Y, Takahashi M et al (2012) Antitumor effect of cetuximab in combination with S-1 in EGFR-amplified gastric cancer cells. Int J Oncol 40(4):975–982. https://doi.org/10.3892/ijo.2011.1279
Nukatsuka M, Saito H, Nakagawa F et al (2012) Combination therapy using oral S-1 and targeted agents against human tumor xenografts in nude mice. Exp Ther Med 3(5):755–762. https://doi.org/10.3892/etm.2012.484
Takahashi T, Emi Y, Oki E et al (2016) Multicenter phase II study of combination therapy with cetuximab and S-1 in patients with KRAS exon 2 wild-type unresectable colorectal cancer previously treated with irinotecan, oxaliplatin, and fluoropyrimidines (KSCC 0901 study). Cancer Chemother Pharmacol 78(3):585–593. https://doi.org/10.1007/s00280-016-3109-4
Eisenhauer EA, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45(2):228–247. https://doi.org/10.1016/j.ejca.2008.10.026
Inuyama Y, Kida A, Tsukuda M et al (2001) Late phase II study of S-1 in patients with advanced head and neck cancer. Gan kagaku ryoho Cancer Chemother 28(10):1381–1390
Saloura V, Cohen EE, Licitra L et al (2014) An open-label single-arm, phase II trial of zalutumumab, a human monoclonal anti-EGFR antibody, in patients with platinum-refractory squamous cell carcinoma of the head and neck. Cancer Chemother Pharmacol 73(6):1227–1239. https://doi.org/10.1007/s00280-014-2459-z
Baselga J, Trigo JM, Bourhis J et al (2005) Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol 23(24):5568–5577. https://doi.org/10.1200/JCO.2005.07.119
Herbst RS, Arquette M, Shin DM et al (2005) Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J Clin Oncol 23(24):5578–5587. https://doi.org/10.1200/JCO.2005.07.120
Ferris RL, Blumenschein G Jr, Fayette J et al (2016) Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375(19):1856–1867. https://doi.org/10.1056/NEJMoa1602252
Cohen EEW, Soulieres D, Le Tourneau C et al (2019) Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 393(10167):156–167. https://doi.org/10.1016/S0140-6736(18)31999-8
Schvartsman G, Peng SA, Bis G et al (2017) Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer 112:90–95. https://doi.org/10.1016/j.lungcan.2017.07.034
Szabados B, van Dijk N, Tang YZ et al (2018) Response Rate to Chemotherapy After Immune Checkpoint Inhibition in Metastatic Urothelial Cancer. Eur Urol 73(2):149–152. https://doi.org/10.1016/j.eururo.2017.08.022
Rossi C, Gilhodes J, Maerevoet M et al (2018) Efficacy of chemotherapy or chemo-anti-PD-1 combination after failed anti-PD-1 therapy for relapsed and refractory Hodgkin lymphoma: a series from Lysa centers. Am J Hematol. https://doi.org/10.1002/ajh.25154
Saleh K, Daste A, Martin N et al (2019) Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer 121:123–129. https://doi.org/10.1016/j.ejca.2019.08.026
Burtness B, Harrington KJ, Greil R et al (2019) Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet 394(10212):1915–1928. https://doi.org/10.1016/S0140-6736(19)32591-7
Hitt R, Irigoyen A, Cortes-Funes H et al (2012) Phase II study of the combination of cetuximab and weekly paclitaxel in the first-line treatment of patients with recurrent and/or metastatic squamous cell carcinoma of head and neck. Ann Oncol 23(4):1016–1022. https://doi.org/10.1093/annonc/mdr367
Vermorken JB, Trigo J, Hitt R et al (2007) Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol 25(16):2171–2177. https://doi.org/10.1200/JCO.2006.06.7447
Acknowledgements
This study did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Hirotoshi Dosaka-Akita received scholarship donations from Taiho Pharmaceutical Company. The remaining co-authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Taguchi, J., Shimizu, Y., Ariga, S. et al. Phase II trial of combination treatment with S-1/cetuximab in patients with platinum-ineligible recurrent and/or metastatic squamous cell carcinoma of the head and neck. Int J Clin Oncol 26, 51–58 (2021). https://doi.org/10.1007/s10147-020-01788-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01788-6