Abstract
Purpose
FOLFOXIRI plus bevacizumab is regarded as a first-line therapeutic option for selected patients with metastatic colorectal cancer (mCRC). Our aim was to assess the efficacy and safety of induction treatment with FOLFOXIRI plus bevacizumab in patients with untreated mCRC harboring UGT1A1 wild (*1/*1), or single-hetero (*1/*6 or *1/*28) genotypes.
Methods
Twelve cycles of FOLFOXIRI plus bevacizumab were administered to patients with untreated mCRC. The primary endpoint was the overall response rate (ORR) assessed by central independent reviewers. Secondary endpoints included time to treatment failure (TTF), progression-free survival (PFS), overall survival (OS), relative dose intensity (RDI), R0 resection rate, and safety. The exploratory objectives were early tumor shrinkage (ETS) and depth of response (DoR).
Results
Of the 47 patients enrolled, 46 and 44 patients were eligible for the safety and efficacy analysis, respectively. The primary endpoint was met. The ORR was 63.6% (95% CI 47.8–77.6). At a median follow-up of 25.4 months, median TTF, PFS, and OS was 8.1, 15.5, and 34.4 months, respectively. The median RDI of 5-fluorouracil, irinotecan, oxaliplatin, and bevacizumab was 72, 69, 62, and 71%, respectively. R0 resection rate was 22.7%. Grade 3 or higher adverse events (≥ 10%) included neutropenia (65.2%), febrile neutropenia (26.1%), leukopenia (23.9%), anorexia (10.9%), nausea (10.9%), and diarrhoea (10.9%). No treatment-related deaths were observed. ETS and DoR were 70.5 and 45.4%, respectively.
Conclusions
FOLFOXIRI plus bevacizumab induction treatment of Japanese patients was shown to be beneficial and manageable, although caution is required since the treatment causes febrile neutropenia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer (CRC) is the third most common malignant neoplasm and the second major cause of cancer death in 2018 worldwide for both sexes [1]. Most patients with metastatic CRC (mCRC) have unresectable disease and cannot be cured. However, long-term survival or even cure is reported to have been attained in 20–50% of patients who underwent complete R0 resection of their metastases [2].
The Gruppo Oncologico Nord Ovest (GONO) group’s phase III TRIBE trial compared FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab in unresectable mCRC patients in Italy [3]. This trial demonstrated that FOLFOXIRI plus bevacizumab increased ORR (65 vs. 53%, p = 0.006) and PFS (12.1 vs. 9.7 months, p = 0.003). An updated analysis on the TRIBE trial reported that OS was prolonged (29.8 vs. 25.8 months, p = 0.03) [4].
The major clinical practice guidelines, including the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guideline 2019, all recommend FOLFOXIRI plus bevacizumab as a first-line therapeutic option for selected patients with mCRC [5,6,7,8]. Only two prospective, single-arm phase II trials of FOLFOXIRI plus bevacizumab have been conducted to assess the safety and efficacy in Japan. One is the QUATTRO study using the GONO-FOLFOXIRI regimen [9]. The other is the JACCRO-CC11 trial using a modified FOLFOXIRI regimen that consisted of oxaliplatin (85 mg/m2), irinotecan (150 mg/m2), l-leucovorin (200 mg/m2), and 5-fluorouracil (2400 mg/m2) [10]. Both studies concluded that FOLFOXIRI plus bevacizumab was effective. The incidence of febrile neutropenia was as low as 5% in modified FOLFOXIRI compared to 21.7% in GONO-FOLFOXIRI. However, mature PFS and OS have not been documented for either of these studies. The recommended doses of FOLFOXIRI also remain controversial.
Adverse events associated with the FOLFOXIRI regimen include higher risks of neutropenia and diarrhoea [11]. Irinotecan especially has significant adverse effects, including myelosuppression and diarrhoea. In Japanese patients, homozygosity for UGT1A1*28 or UGT1A1*6 and heterozygosity for both UGT1A1*6 and UGT1A1*28 are associated with severe irinotecan-related neutropenia [12, 13].
Thus, we conducted a single-arm, multicenter, phase II study to assess the efficacy and safety of induction treatment with FOLFOXIRI plus bevacizumab in patients with untreated mCRC harboring UGT1A1 wild (*1/*1) or single-hetero (*1/*6 or *1/*28) genotypes. In our study, sequential treatment with the remaining drugs was continued after termination of protocol therapy or discontinuation of oxaliplatin and/or irinotecan at the investigator’s discretion.
Patients and methods
Study design
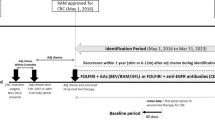
The Bevacizumab plus Triplet (BeTRI) study was a multi-site, open-label, single-arm, phase II clinical trial. This trial was registered on the UMIN Clinical Trials Registry (UMIN000017102), followed by Japan Registry of Clinical Trials (jRCTs061180021).
Patients
Patients who met the following criteria were enrolled in this study: aged between 20 and 70 years; histologically confirmed adenocarcinoma of the colon or rectum; unresectable or recurrent CRC patient; no prior chemotherapy, immunotherapy, or radiation therapy (but can be enrolled 6 months after the date of completion of adjuvant chemotherapy); one or more measurable lesions; ECOG PS of 0 or 1; patients harbored UGT1A1 wild (*1/*1) or single-heterozygous (*1/*6 or *1/*28) genotypes. Patients were ineligible if they had severe, uncontrolled organ or metabolic dysfunction.
Endpoints
The primary endpoint was the objective response rate (ORR) assessed by central independent reviewers. Secondary endpoints were TTF and PFS, OS, R0 resection rate, relative dose intensity (RDI), and safety. TTF represented the time from the initial day of protocol therapy to the first day when we observed any of the following events: discontinuation of protocol therapy; initial progression; death with any cause. PFS was defined as the time from the initial day of protocol therapy to the initial progression day or death for any reason. The exploratory objectives were early tumor shrinkage (ETS) and depth of response (DoR).
Treatment and evaluation
We defined protocol therapy as 12 cycles of FOLFOXIRI plus bevacizumab induction treatment consisting of a 30–90-min infusion of bevacizumab at a dose of 5 mg/kg, a 90-min infusion of irinotecan at a dose of 165 mg/m2, a 120-min infusion of oxaliplatin at a dose of 85 mg/m2 and a concomitant 120-min infusion of l-leucovorin at a dose of 200 mg/m2. These were followed by a 48-h continuous infusion of 5-fluorouracil to a total dose of 3200 mg/m2. Cycles were repeated every 14 days.
The chemotherapy was continued until disease progression, an unacceptable adverse event, tumor resection, a delay of more than 29 days due to an adverse event, reduction of 5-fluorouracil dose to less than 2000 mg/m2, or consent withdrawal. In cases of prespecified adverse events, treatment modification was permitted according to the study protocols (Supplementary Tables 1 and 2). If oxaliplatin and/or irinotecan was discontinued at the investigator's discretion, sequential treatment consisting of the remaining drugs was continued.
We analyzed safety with a safety analysis set (SAS) in which patients received at least one cycle of protocol therapy. Efficacy was analyzed with a full analysis set (FAS) consisting of eligible patients. Based on the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1, central independent reviewers assessed response and progression, referring to CT or MRI taken every 8 weeks. ETS was defined as a reduction of at least 20% in the sum of the longest diameters of target lesions at week 8 compared with baseline. DoR was defined as the relative change in the sum of the longest diameters of RECIST target lesions at the nadir, in the absence of new lesions or progression of non-target lesions, when compared with baseline. Adverse events were evaluated according to CTCAE version 4.0.
Sample size
We assumed the expected response rate to be 70% and the threshold response rate to be 45%, with a two-sided α error of 0.05 and a power of 90%. The expected response rate was grounded in the results of the GONO and TRIBE trials, of which the confirmed ORRs were 65 and 77% for FOLFOXIRI plus bevacizumab, respectively [3, 14]. We also referred to the threshold response rate reported by the NO16966 and TRIBE trials, of which the confirmed ORRs were 34 and 53% for doublet plus bevacizumab, respectively [3, 15]. We estimated a minimum of 40 patients, but we planned to enrol 45 or more patients, taking account of dropouts and withdrawals.
Statistical analyses
We concluded that the treatment could be regarded as promising if the lower limit of the 95% CI exceeded 45%. For the evaluation of the ORR and ETS, we calculated the 95% CI of the rate using the Clopper–Pearson method with F distribution. We used the Kaplan–Meier method to estimate TTF, PFS, and OS. Statistical analyses were carried out using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) and R × 64 version 3.5.2.
Results
Patients
Between April 2015 and May 2017, a total of 47 patients were enrolled from 16 Japanese study sites. One patient was excluded from the study because of a deviation of the inclusion criteria. Two more patients were removed after the central reviewers determined there were no measurable lesions. The numbers in the SAS and FAS were, therefore, 46 and 44, respectively (Supplementary Fig. 1).
Table 1 shows patient characteristics in the SAS. There were twenty-six male and twenty female patients with a median age of 58 years (range 29–68 years). ECOG PS scores of 0 and 1 were 76% and 24%, respectively. The site of the primary tumor was right colon in 10 (22%) patients, left colon in 16 (35%), and rectum in 20 (43%) patients. Resection rate of the primary tumor was 37%. Seventeen (37%), 24 (52%) and 5 (11%) patients had RAS wild-type, RAS mutated, and RAS unknown tumor, respectively.
Study treatment
All 46 enrolled patients received FOLFOXIRI plus bevacizumab. The commencement of second cycle treatment was delayed in 33 (72%) patients. In addition, the dose level was reduced in the second cycle in 23 (50%) patients. Meanwhile, twelve (26%) patients completed the second cycle treatment as scheduled without dose modification.
A total of 27 (59%) patients completed twelve cycles of protocol therapy. Table 2 shows the details of the treatment after protocol therapy. At the twelfth cycle, 18 (39.1%) patients received FOLFOXIRI plus bevacizumab, six (13.0%) patients received FOLFIRI plus bevacizumab, two (4.3%) patients received FOLFIRI, and one (2.2%) patient received infusional 5-fluorouracil and leucovorin plus bevacizumab. The protocol therapy was discontinued in 9 (20%) patients due to adverse events, in 8 (17%) patients due to surgery, in one (2%) patient due to progressive disease, and in one (2%) patient due to withdrawal of consent. Of the nine patients for whom protocol therapy was discontinued due to adverse events, eight patients resumed chemotherapy, and their treatments (number of patients) were as follows: FOLFOXIRI plus bevacizumab (3); FOLFOXIRI (1); FOLFIRI plus bevacizumab (2); Capecitabine plus bevacizumab (1); and 5-fluorouracil and leucovorin plus bevacizumab (1).
Although second-line treatments were not specified by the protocol, we requested that treatment administered after progression of protocol therapy and treatment with drugs not included in the protocol therapy (i.e. second-line treatments) must be reported. Second-line chemotherapy (cases per 16 patients total) were as follows: FOLFIRI plus anti-VEGF/VEGFR (4); FOLFOXIRI plus or minus bevacizumab (3); anti-EGFR plus or minus irinotecan (3); capecitabine plus or minus bevacizumab (3); irinotecan plus tegafur/gimeracil/oteracil (1); regorafenib (1); and trifluridine/tipiracil (1) (Supplementary Table 4).
In the FAS, the median relative dose intensity of 5-fluorouracil, irinotecan, oxaliplatin, and bevacizumab was 72, 69, 62 and 71%, respectively. The median number of cycles administered per patient as FOLFOXIRI plus or minus bevacizumab was eight (range 2–12).
Efficacy
The efficacy results are summarized in Table 3. Because CR and PR were achieved in 1 (2.3%) patient and 27 (61.4%) patients, respectively, the ORR was 63.6% (95% CI 47.8–77.6). The numbers (%) of patients with stable disease (SD) and with progressive disease (PD) were 14 (31.8%) and 2 (4.5%), respectively. In addition, the ORR at week 8 was 54.5%. ETS was 70.5% (95% CI 54.8–83.2). A waterfall plot of maximum tumor reduction rate is presented in Fig. 1. Median DoR was 45.4% (95% CI 37.8–49.9).
The median follow-up period was 25.4 months (ranging from 6 to 47 months). Median TTF was 8.1 months (95% CI 5.3–10.1) based on 38 events (86.4%) among 44 patients (Fig. 2a). Median PFS was 15.5 months (95% CI 11.5–23.4) based on 28 events (63.6%) among 44 patients (Fig. 2b). Median OS was 34.4 months (95% CI 26.4 not reached) based on 19 deaths among 44 patients (43.2%) (Fig. 2c).
Subgroup analyses by primary tumor sidedness and RAS mutational status
We evaluated the activity of FOLFOXIRI plus bevacizumab in an ad hoc analysis on the basis of primary tumor sidedness or RAS status (Table 3). Following classification of the 44 patients in the FAS on the basis of primary tumor sidedness and RAS mutation status, left-sided colon tumors were associated with a higher frequency of mutated RAS tumors than right-sided colon tumors (47.7 vs. 6.8%) (Supplementary Table 3). In stratification by sidedness, the ORR was numerically higher in right-sided colon tumors compared with left-sided ones (70.0 vs. 61.8%, respectively). As for ETS and DoR, there was no difference between the two sides.
In stratification by RAS status, the ORR was numerically higher in RAS wild-type tumors compared with RAS mutated ones (73.3 vs. 62.5%). ETS and median DoR were 73.3 and 47.6% in RAS wild-type tumors and 70.8 and 45.2% in RAS mutated tumors, respectively.
When analyzed on the basis of primary tumor sidedness, median PFS was 24.1 months (95% CI 11.5–26.2) and 14.9 months (95% CI 10.1–22.9) in right- and left-sided colon tumor cases, respectively, and the two survival curves intersected in the follow-up period (Supplementary Fig. 2a). Median OS was 26.4 months (95% CI 26.2 not reached) and 34.4 months (95% CI 23.6 not reached) in right- and left-sided colon tumor cases, respectively (Supplementary Fig. 2c). When analyzed according to RAS mutational status, median PFS was 13.2 months (95% CI 11.4–24.1) and 17.9 months (95% CI 13.7–26.2) in RAS wild-type and mutated tumor cases, respectively (Supplementary Fig. 2b), and the two survival curves crossed. Median OS was 37.1 months (95% CI 23.6 not reached) and 29.8 months (95% CI 26.2 not reached) in RAS wild-type and mutated tumor cases, respectively (Supplementary Fig. 2d).
Conversion surgery
Fourteen patients (31.8%) had conversion surgery performed with curative intent. Nine of these patients underwent surgery during the protocol therapy, and two patients did after the end of protocol therapy. The remaining three patients did during sequential treatment following the completion of protocol therapy. The median time to surgery from the initial day of protocol therapy was 182.5 days (range 87–316 days).
R0 resection was performed in 10 (22.7%) patients. Five of these patients were treated with 12 cycles of protocol therapy (two patients) or sequential treatment (three patients). When we examined the presence of ETS in ten patients with R0 resection, eight patients had ETS and two patients had no ETS. The surgical procedures (number of patients) performed on the four patients who could not achieve R0 resection included primary resection (3) and liver and lung resection (1).
Toxicity
All observed adverse events in the SAS are summarized in Table 4. Treatment-related grade 3 or 4 adverse events occurring in at least 10% of patients were as follows: neutropenia (65.2%); febrile neutropenia (26.1%); leukopenia (23.9%); nausea (10.9%); anorexia (10.9%); and diarrhea (10.9%). Two (16.7%) of the 12 patients who developed febrile neutropenia were treated with G-CSF. No new adverse events were observed compared with previous historical studies.
In total, 14 serious adverse events occurred in 11 patients (23.9%). Five of these adverse events were independent of protocol therapy. The remaining adverse events included infections (4), a thromboembolic event (1), an anastomosis fistula (1), ileus (1), nausea (1), and fever (1). All patients recovered from the serious adverse events at the end of the study. No treatment-related deaths occurred in the present study.
Discussion
Our BeTRI study confirmed the efficacy and safety of GONO-FOLFOXIRI plus bevacizumab in Japanese mCRC patients harboring UGT1A1 wild (*1/*1) or single-heterozygous (*1/*6 or *1/*28) genotypes.
Right-sided colon tumors are associated with a poorer prognosis compared with left-sided tumors [16, 17]. Considering only patients with previously untreated mCRC receiving first-line doublet chemotherapy plus or minus bevacizumab, Loupakis et al. observed that ORR and PFS were statistically significantly higher in patients with left-sided tumors [16]. Interestingly, our ad hoc analysis showed that FOLFOXIRI plus bevacizumab treatment produced a numerically higher ORR, TTF, and PFS in right-sided colon tumor cases (compared with left-sided tumors). In right-sided colon tumor cases, ORR was the same, irrespective of RAS mutational status. This finding supports the post hoc analysis of the TRIBE trial which found that FOLFOXIRI plus bevacizumab may be able to efficiently counteract the intrinsic aggressiveness of right-sided colon tumors [18].
FOLFOXIRI plus bevacizumab with an expected ORR of around 70% is an attractive strategy for conversion chemotherapy. Interestingly, conversion surgery (31.8%) and R0 resection (22.7%) rate in the present study were consistent with previous studies [9, 10, 14], despite the decreased dose intensity. The median time to surgery from the initial day of protocol therapy was 182.5 days (range 87–316 days). Of the 14 patients who underwent surgery, the chemotherapy regimen immediately prior to surgery was FOLFOXIRI (n = 11), FOLFIRI (n = 1), and 5-fluorouracil and leucovorin (n = 2). Among the 10 patients who had undergone R0 resection, 50% of patients were treated with 12 or more cycles of protocol therapy. The ten patients who underwent R0 resection included two patients without ETS. This finding supports previously published meta-regression analysis data suggesting that a high number of chemotherapy cycles (close to 12) is associated with conversion surgery [19]. Therefore, a sufficient treatment time period may be required for R0 resection in unresectable or recurrent CRC.
Considering the safety profile of this drug regimen, 26.1% of Japanese patients developed febrile neutropenia in our BeTRI study, which was consistent with the results of the QUATTRO study. It should be noted that only two (16.7%) of 12 patients were treated with G-CSF as a primary treatment or secondary prophylaxis of febrile neutropenia. The second cycle of treatment was performed without modification of dose or delay in 12 (26%) patients. The median relative dose intensities of 5-fluorouracil, irinotecan, oxaliplatin, and bevacizumab were 72, 69, 62, and 71%, respectively. Compared with earlier studies [9, 14], the frequency of G-CSF use was extremely low. This may be related to the lower dose intensities in comparison with previous studies. The observed incidence of grade 3 or more diarrhea was low (10.9%) compared with the two GONO-FOLFOXIRI plus bevacizumab studies (14–18.8%) [4, 14], and was comparable with the QUATTRO study [9]. The major differences from the TRIBE trial was that target patients who harbored UGT1A1*1/*1, *1/*6, or *1/*28 were included in our BeTRI and QUATTRO studies. This inclusion criterion may be the reason for the lower frequency of diarrhea. Our results indicate that the GONO-FOLFOXIRI regimen could be managed with appropriate dose reductions and treatment delays in Japanese patients harboring UGT1A1 wild (*1/*1), or single-hetero (*1/*6 or *1/*28) genotypes.
In clinical trials, chemotherapy is historically administered until disease progression, unacceptable toxicities, or patients’ refusal. Oxaliplatin often causes cumulative neurotoxicity before clinical progression [20]. NCCN and ASCO guidelines recommend discontinuation of oxaliplatin from FOLFOX or CAPEOX three–four months after initiation of treatment or sooner for unacceptable neurotoxicity, with other drugs in the regimen maintained until time of tumor progression [6, 7]. In contrast, continuation of FOLFIRI induction treatment is recommended for at least as long as tumor shrinkage continues, or disease stabilization is maintained with tolerable toxicities [5]. In the TRIBE trial, 76% of patients received secondary treatment, of which 63% were on irinotecan-containing regimens [4]. In our BeTRI study, a sequential treatment following FOLFOXIRI plus bevacizumab was not intentionally defined as maintenance treatment with 5-fluorouracil and leucovorin plus bevacizumab. A total of 27 (59%) patients completed twelve cycles of protocol therapy. In fact, at the twelfth cycle, 18 (39.1%) patients received FOLFOXIRI plus bevacizumab, six (13.0%) patients received FOLFIRI plus bevacizumab, two (4.3%) patients received FOLFIRI, and one (2.2%) patient received infusional 5-fluorouracil and leucovorin plus bevacizumab. It should be noted that a FOLFIRI regimen was opted for eight (30.8%) patients. Moreover, R0 resection was performed in 10 (22.7%) patients including five patients treated with more than 12 cycles. These may have impacted on the encouraging lengths of 15.5 months for PFS and 37.1 months for OS. Taking the above into account, FOLFIRI appears to be suitable as a sequential treatment after three to four months of FOLFOXIRI or sooner for unacceptable neurotoxicity.
The current study had several potential limitations. A major weakness was that our trial was a single-arm, small sample size study with no comparators. Second, data on BRAF mutational status were not available. Finally, the follow-up time was short for evaluation of OS. In the future, appropriate sequential treatment strategies following FOLFOXIRI induction treatment should be considered.
In conclusion, our BeTRI study demonstrated that GONO-FOLFOXIRI plus bevacizumab were highly beneficial and manageable with toxicities in selected Japanese patients with good performance status who can tolerate intensive treatment.
References
Bray F, Ferlay J, Soerjomataram I et al (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
House MG, Ito H, Gonen M et al (2010) Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1600 patients during two decades at a single institution. J Am Coll Surgeons 210(5):744–752. https://doi.org/10.1016/j.jamcollsurg.2009.12.040 (752–745)
Loupakis F, Cremolini C, Masi G et al (2014) Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 371(17):1609–1618. https://doi.org/10.1056/NEJMoa1403108
Cremolini C, Loupakis F, Antoniotti C et al (2015a) FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol 16(13):1306–1315. https://doi.org/10.1016/s1470-2045(15)00122-9
Van Cutsem E, Cervantes A, Adam R et al (2016) ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27(8):1386–1422. https://doi.org/10.1093/annonc/mdw235
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology—colon cancer, version 4.2020. Accessed 17 July 2020
Chiorean EG, Nandakumar G, Fadelu T et al (2020) Treatment of patients with late-stage colorectal cancer: ASCO Resource-Stratified Guideline. JCO Global Oncol 6:414–438. https://doi.org/10.1200/jgo.19.00367
Hashiguchi Y, Muro K, Saito Y et al (2020) Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol 25(1):1–42. https://doi.org/10.1007/s10147-019-01485-z
Oki E, Kato T, Bando H et al (2018) A multicenter clinical phase II study of FOLFOXIRI plus Bevacizumab as first-line therapy in patients with metastatic colorectal cancer: QUATTRO Study. Clin Colorectal Cancer 17(2):147–155. https://doi.org/10.1016/j.clcc.2018.01.011
Satake H, Sunakawa Y, Miyamoto Y et al (2018) A phase II trial of 1st-line modified-FOLFOXIRI plus bevacizumab treatment for metastatic colorectal cancer harboring RAS mutation: JACCRO CC-11. Oncotarget 9(27):18811–18820. https://doi.org/10.18632/oncotarget.24702
Shui L, Wu YS, Lin H et al (2018) Triplet Chemotherapy (FOLFOXIRI) plus Bevacizumab versus doublet chemotherapy (FOLFOX/FOLFIRI) plus Bevacizumab in conversion therapy for metastatic colorectal cancer: a meta-analysis. Cell Physiol Biochem 48(5):1870–1881. https://doi.org/10.1159/000492508
Ando Y, Saka H, Ando M et al (2000) Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res 60(24):6921–6926
Minami H, Sai K, Saeki M et al (2007) Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: roles of UGT1A1*6 and *28. Pharmacogenet Genomics 17(7):497–504. https://doi.org/10.1097/FPC.0b013e328014341f
Masi G, Loupakis F, Salvatore L et al (2010) Bevacizumab with FOLFOXIRI (irinotecan, oxaliplatin, fluorouracil, and folinate) as first-line treatment for metastatic colorectal cancer: a phase 2 trial. Lancet Oncol 11(9):845–852. https://doi.org/10.1016/s1470-2045(10)70175-3
Cassidy J, Clarke S, Diaz-Rubio E et al (2008) Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 26(12):2006–2012. https://doi.org/10.1200/jco.2007.14.9898
Loupakis F, Yang D, Yau L et al (2015) Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer I. https://doi.org/10.1093/jnci/dju427
Petrelli F, Tomasello G, Borgonovo K et al (2017) Prognostic survival associated with left-sided vs right-sided colon cancer: A systematic review and meta-analysis. JAMA Oncol 3(2):211–219. https://doi.org/10.1001/jamaoncol.2016.4227
Cremolini C, Loupakis F, Antoniotti C et al (2015b) Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the GruppoOncologico del Nord Ovest. Ann Oncol 26(6):1188–1194. https://doi.org/10.1093/annonc/mdv112
Tomasello G, Petrelli F, Ghidini M et al (2017) FOLFOXIRI plus Bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: A systematic review and pooled analysis. JAMA Oncol 3(7):e170278. https://doi.org/10.1001/jamaoncol.2017.0278
Kidwell KM, Yothers G, Ganz PA et al (2012) Long-term neurotoxicity effects of oxaliplatin added to fluorouracil and leucovorin as adjuvant therapy for colon cancer: results from National Surgical Adjuvant Breast and Bowel Project trials C-07 and LTS-01. Cancer 118(22):5614–5622. https://doi.org/10.1002/cncr.27593
Acknowledgments
We thank the patients who participated in this study and their families, the physicians and the medical staffs of the 16 sites registered in this study, and Dr. Hisayuki Endo, Dr. Yasuo Iwamoto, Dr. Takao Hinoi, and Dr. Satoshi Teramukai for comprising the independent data monitoring committee. We also thank Dr. Kunihisa Miyakawa and Dr. Masaki Matsusako for the central review. We acknowledge and appreciate the data analyses work carried out by Ms. Yoko Nakagawa and Mr. Hiromi Tsukada. We also appreciate the work of Mr. Tasuku Inaji in his duty as study manager in the Translational Research Center for Medical Innovation (TRI).
Funding
This study was funded by Yakult Honsha Co., Ltd under contract. Yakult Honsha Co., Ltd played no role in the design, collection, analysis, or interpretation of the data, or writing of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors read and approved the final manuscript, and author contributions are in line with ICMJE guidelines. Substantial contributions: Planning and designing the study: KS, TN, TK. Acquisition of data: KS, TY, JN, TM, YY, TS, HN, IM, TF, MM, RY, KN, HT, TN, YK, MK, KT, MI, TK, TN. Writing the manuscript: KS, TY, TK, TN.
Corresponding author
Ethics declarations
Conflict of interest
KS has received speaker’s honoraria from Chugai Pharmaceutical Co., Ltd., outside the submitted work; MK has received research funding from Chugai Pharmaceutical Co., Ltd., Yakult Honsha Co., Ltd., outside the submitted work. TN has received honoraria from Taiho Pharmaceutical Co., Ltd., and research funding from Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Bristol Myers Squibb K.K., Eli Lilly Japan K.K., Daiichi Sankyo Co., Ltd., MSD K.K., Sumitomo Dainippon pharma Co., Ltd., outside the submitted work. TY, JN, TM, YY, TS, HN, IM, TF, MM, RY, KN, HT, TN, YK, KT, MI and TK declares no conflicts of interest.
Ethical approval
This study was conducted by the Translational Research Center for Medical Innovation (TRI) in accordance with the Declaration of Helsinki and Clinical Trials Act in Japan. The study protocol was approved by the Institutional Review Board of each participating institution and Certified Review Board. All patients provided written informed consent. This study was registered with UMIN Clinical Trial Registry (Trial Identifier UMIN000017102), the Japan Registry of Clinical Trials (jRCTs061180021), and ClinicalTrials.gov (NCT02497157).
Informed consent
Informed consent was obtained from all individual participants included in this study.
Availability of data and material
The datasets generated during the current study are not publicly available because the informed consent form signed by the participants did not address an individual data sharing statement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Shinozaki, K., Yamada, T., Nasu, J. et al. A phase II study of FOLFOXIRI plus bevacizumab as initial chemotherapy for patients with untreated metastatic colorectal cancer: TRICC1414 (BeTRI). Int J Clin Oncol 26, 399–408 (2021). https://doi.org/10.1007/s10147-020-01811-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-020-01811-w