Abstract
Background
The efficacy of primary prophylactic granulocyte colony-stimulating factor (G-CSF) in preventing febrile neutropenia (FN) in patients treated with docetaxel, cisplatin, and 5-fluorouracil (TPF) chemotherapy remains controversial. We compared the incidence of FN in patients treated with and without primary prophylactic G-CSF.
Methods
We performed a retrospective analysis of 142 patients with locally advanced head and neck or esophageal cancer treated with TPF between January 2009 and March 2017. Among them, 116 patients started TPF without primary prophylactic G-CSF (control group) while 26 patients were given primary prophylactic G-CSF from day 7 of the first cycle of TPF (prophylactic group).
Results
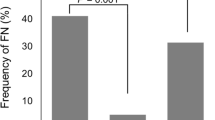
The incidence of grade 4 neutropenia during the first cycle of TPF was significantly higher in the control group than in the prophylactic group [58.6% (n = 68) vs. 30.8% (n = 8), p = 0.02]. However, the incidence of FN in the first cycle was not significantly different between the two groups [32 patients (27.5%) in the control group and 8 patients (30.8%) in the prophylactic group (p = 0.62)]. In addition, the mean relative dose intensity throughout all cycles of TPF, as well as the survival time and response after TPF, were also not significantly different between the two groups.
Conclusions
Primary prophylactic G-CSF from day 7 of the first cycle of TPF did not reduce the incidence of FN. Our findings suggest that the timing of primary prophylactic G-CSF, as recommended by the American Society of Clinical Oncology guidelines, should be modified to reduce the incidence of FN in TPF.

Similar content being viewed by others
References
Posner MR, Hershock DM, Blajman CR et al (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 357:1705–1715
Vermorken JB, Remenar E, van Herpen C et al (2007) Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 357:1695–1704
Hara H, Tahara M, Daiko H et al (2013) Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci 104:1455–1460
Yokota T, Kato K, Hamamoto Y et al (2016) Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer 115:1328–1334
Van Cutsem E, Moiseyenko VM, Tjulandin S et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24:4991–4997
Pointreau Y, Garaud P, Chapet S et al (2009) Randomized trial of induction chemotherapy with cisplatin and 5-fluorouracil with or without docetaxel for larynx preservation. J Natl Cancer Inst 101:498–506
Smith TJ, Bohlke K, Lyman GH et al (2015) Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 33(28):3199–3212
Izawa N, Onozawa Y, Hikosaka T et al (2015) Efficacy and feasibility of docetaxel, cisplatin, and 5 fluorouracil induction chemotherapy for locally advanced head and neck squamous cell carcinoma classified as clinical nodal stage N2c, N3, or N2b with supraclavicular lymph node metastases. Int J Clin Oncol 20:455–462
Kawahira M, Yokota T, Hamauchi S et al (2017) Survival benefit of adding docetaxel, cisplatin, and 5-fluorouracil induction chemotherapy to concurrent chemoradiotherapy for locally advanced nasopharyngeal carcinoma with nodal Stage N2–3. Jpn J Clin Oncol 20:1–8
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transpl 48:452–458
Linot B, Augereau P, Breheret R et al (2014) Efficacy and safety of early G-CSF administration in patients with head and neck cancer treated by docetaxel-cisplatin and 5-fluorouracil (DCF protocol): a retrospective study. Support Care Cancer 22(10):2831–2837
Schuman SI, Lambrou N, Robson K et al (2009) Pegfilgrastim dosing on same day as myelosuppressive chemotherapy for ovarian or primary peritoneal cancer. J Support Oncol 7:225–228
Whitworth JM, Matthews KS, Shipman KA et al (2009) The safety and efficacy of day 1 versus day 2 administration of pegfilgrastim in patients receiving myelosuppressive chemotherapy for gynecologic malignancies. Gynecol Oncol 112:601–604
Filgrastim [package insert] (2013) Tokyo: Nippon Kayaku Inc
Molineux G, Kinstler O, Briddell B et al (1999) A new form of Filgrastim with sustained duration in vivo and enhanced ability to mobilize PBPC in both mice and humans. Exp Hematol 27:1724–1734
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kentaro Yamazaki has received honoraria from Yakult. The other authors have no conflict of interests to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of our institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. For this type of study, formal consent is not required.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10147_2018_1306_MOESM1_ESM.docx
Supplementary material 1 Fig. S-1 Kaplan–Meier plot shows the rate of progression-free survival of patients in the control and prophylactic groups (n = 142). Fig. S-2 Kaplan–Meier plot shows the rate of overall survival of patients in the control and prophylactic groups (n = 142) (DOCX 63 KB)
About this article
Cite this article
Kawahira, M., Yokota, T., Hamauchi, S. et al. Primary prophylactic granulocyte colony-stimulating factor according to ASCO guidelines has no preventive effect on febrile neutropenia in patients treated with docetaxel, cisplatin, and 5-fluorouracil chemotherapy. Int J Clin Oncol 23, 1189–1195 (2018). https://doi.org/10.1007/s10147-018-1306-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-018-1306-3