Abstract
Background
We evaluated the efficacy and feasibility of docetaxel, cisplatin, and 5-fluorouracil (TPF) induction chemotherapy followed by concurrent chemoradiotherapy (CRT) for locally advanced head and neck squamous cell carcinoma (HNSCC) with a high risk of distant metastases compared with CRT alone.
Methods
We retrospectively analyzed 29 HNSCC patients with clinical nodal stage N2c, N3, or N2b disease and supraclavicular lymph node metastases receiving CRT alone (CRT group; n = 16) or TPF induction chemotherapy followed by CRT (TPF group; n = 13) between April 2008 and May 2012.
Results
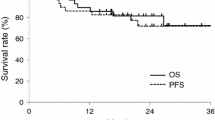
The median follow-up periods were 14.5 (range 5.0–65.0) and 25.0 (range 14.0–32.0) months for CRT and TPF groups, respectively. A greater proportion of patient characteristics in the CRT group had advanced T and N stages. The overall response rate to induction TPF was 50.0 %; grade 3–4 toxicities included neutropenia, febrile neutropenia, anorexia, and hyponatremia. Complete response rates after CRT completion were 55.5 % in the TPF and 42.9 % in the CRT group; median overall survival was not reached in the TPF group and was 14.0 months in the CRT group (p = 0.037). Multivariate analysis revealed that induction TPF and T stage were independent prognostic factors [hazard ratio (HR) = 0.196; 95 % confidence interval (CI) 0.043–0.898; p = 0.036, HR = 9.966; 95 % CI 2.270–43.75; p = 0.002, respectively).
Conclusion
TPF followed by CRT is tolerated and may be an option for the treatment of locally advanced stage N2c, N3, or N2b HNSCC.


Similar content being viewed by others
References
Jemal A, Bray F, Center MM et al (2011) Global cancer statistics. CA Cancer J Clin 6:69–90
Vokes EE, Weichselbaum RR, Lippman SM et al (1993) Head and neck cancer. N Engl J Med 328:184–194
Denis F, Garaud P, Bardet E et al (2004) Final results of the 94–01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J Clin Oncol 22:69–76
Adelstein DJ, Li Y, Adams GL et al (2003) An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J Clin Oncol 21:92–98
Lefebvre JL, Chevalier D, Luboinski B et al (1996) Larynx preservation in pyriform sinus cancer: preliminary results of a European Organization for Research and Treatment of Cancer Phase III trial. EORTC Head and Neck Cancer Cooperative Group. J Natl Cancer Inst 88:890–899
Pignon JP, Bourhis J, Domenge C et al (2000) Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. Lancet 355:949–955
Posner MR, Hershock DM, Blajman CR et al (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 357:1705–1715
Vermorken JB, Remenar E, van Herpen C et al (2007) Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 357:1695–1704
Forastiere AA, Goepfert H, Maor M et al (2003) Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med 349:2091–2098
Pignon JP, Al Maître, Maillard E et al (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92:4–14
Brockstein B, Haraf DJ, Rademaker AW et al (2004) Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: a 9-year, 337-patient, multi-institutional experience. Ann Oncol 15:1179–1186
Cerezo L, Millan I, Torre A et al (1992) Prognostic factors for survival and tumor control in cervical lymph node metastases from head and neck cancer: a multivariate study of 492 cases. Cancer 69:1224–1234
de Bree R, Deurloo EE, Snow GB et al (2000) Screening for distant metastases in patients with head and neck cancer. Laryngoscope 110:397–401
Garavello W, Ciardo A, Spreafico R et al (2006) Risk factors for distant metastases in head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 132:762–766
León X, Quer M, Orús C et al (2000) Distant metastases in head and neck cancer patients who achieved loco-regional control. Head Neck 22:680–686
Matsuo JM, Patel SG, Singh B et al (2003) Clinical nodal stage is an independently significant predictor of distant failure in patients with squamous cell carcinoma of the larynx. Ann Surg 238:412–421
Hara H, Tahara M, Daiko H et al (2013) Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci 104:1455–1460
Ura T, Nagase M, Fujii H et al (2010) Feasibility study of preoperative docetaxel (D), cisplatin (C), and fluorouracil (F) in esophageal cancer. In: Abstracts of the 2010 ASCO Gastrointestinal Cancers Symposium (abstr 81). http://meetinglibrary.asco.org/content/1857-72. Accessed 13 Aug 2014
Haddad R, O’Neill A, Rabinowits G et al (2013) Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol 14:257–264
Cohen EE, Karrison T, Kocherginsky M et al (2012) DeCIDE: a phase III randomized trial of docetaxel (D), cisplatin (P), 5-fluorouracil (F) (TPF) induction chemotherapy (IC) in patients with N2/N3 locally advanced squamous cell carcinoma of the head and neck (SCCHN). J Clin Oncol 30:356s (Suppl 15; abstr 5500)
Hitt R, Grau JJ, López-Pousa A et al (2014) A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol 25:216–225
Conflict of interest
Narikazu Boku received a research grant from Taiho and Chugai and lecture fees from Taiho, Chugai, and Shionogi. Tomoya Yokota serves as a consultant to AstraZeneca and receives lecture fees from Merck Serono and Bristol-Myers Squibb. The other authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Izawa, N., Onozawa, Y., Hikosaka, T. et al. Efficacy and feasibility of docetaxel, cisplatin, and 5-fluorouracil induction chemotherapy for locally advanced head and neck squamous cell carcinoma classified as clinical nodal stage N2c, N3, or N2b with supraclavicular lymph node metastases. Int J Clin Oncol 20, 455–462 (2015). https://doi.org/10.1007/s10147-014-0749-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-014-0749-4