Abstract
Background
Our previous phase-3 study (TTCC 2503) failed to show overall survival advantage of 2 induction chemotherapy (IC) regimens followed by standard concurrent chemoradiotherapy (CRT) over CRT alone in patients with unresectable locally advanced head and neck squamous-cell carcinoma (LAHNSCC). This study described the long-term survival of those patients.
Materials and methods
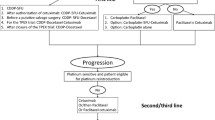
Long-term follow-up study of patients with untreated LAHNSCC assigned to IC (three cycles), with either docetaxel, cisplatin and 5-fluorouracil (TPF arm) or cisplatin and 5-fluorouracil (PF arm), followed by CRT, or CRT alone, included in the previous TTCC 2503 trial.
Results
In the intention-to-treat population (n = 439), the median OS times were 25.4 (95% CI, 16.8–34.4), 26.2 (95% CI, 18.2–36.6) and 25.4 months (95% CI, 17.4–36.0) in the TPF-CRT, PF-CRT and CRT arms, respectively (log-rank p = 0.51). In the per-protocol population (n = 355), patients with larynx–hypopharynx primary tumors treated with IC (TPF or PF) followed by CRT had a longer median PFS than those who received CRT alone. Moreover, patients with ECOG 0 treated with IC (TPF or PF) followed by CRT had a better TTF than those with CRT alone. There were no statistically significant differences in terms of OS, PFS or TTF, according to the tumor load or affected nodes.
Conclusion
After a long follow-up, the TTCC 2503 trial failed to show the benefit of IC-CRT in unresectable LAHNSCC regarding the primary end point. However, fit patients with ECOG 0 and primary larynx–hypopharyngeal tumors may benefit from the use of IC if administered by an experienced team.
ClinicalTrials.gov identifier NCT00261703




Similar content being viewed by others
References
Pignon JP, le Maitre A, Maillard E, Bourhis J, MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17346 patients. Radiother Oncol. 2009;92:4–14.
El-Naggar AK, Chan JKC, Takata T, Slootweg PJ. WHO Classification of head and neck tumours. WHO classification of head and neck tumours. 4th ed. Lyon: International Agency for Research on Cancer; 2017.
Ghi MG, Paccagnella A. Therapeutic intensification and induction chemotherapy for high-risk locally advanced squamous cell carcinoma. Curr Treat Options Oncol. 2019;20:2.
Gillison ML, Chaturvedi AK, Anderson WF, Fakhry C. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J Clin Oncol. 2015;33:3235–42.
Hitt R, Grau JJ, Lopez-Pousa A, Berrocal A, Garcia-Giron C, Irigoyen A, et al. A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol. 2014;25:216–25.
Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, et al. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349:2091–8.
Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15.
Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–704.
Ghi MG, Paccagnella A, Ferrari D, Foa P, Alterio D, Codeca C, et al. Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial Ann Oncol. 2017;28:2206–12.
Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol. 2014;32:2735–43.
Haddad R, O'Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 2013;14:257–64.
Hitt R, Mesia R, Grau JJ, Iglesias L, Del Barco E, Lozano A, et al. Randomized phase III trial of induction chemotherapy (ICT) with docetaxel-cisplatin-5fluorouracil (DCF) followed by cisplatin-radiotherapy (CRT) or cetuximab-radiotherapy (CetRT) in patients (pts) with locally advanced unresectable head and neck cancer (LAUHNC). J Clin Oncol. 2016;34(15):6001.
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tan PF, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35.
Acknowledgements
The authors thank the patients and the medical and nursing staff from all the participating institutions. Dr. Carral (Hospital Lucus Augusti, Lugo, Spain) and Dr. Pérez-Segura (Hospital Clínico San Carlos, Madrid, Spain). Statistics and Data Management: Pivotal. Support for third-party writing assistance for this manuscript was provided by Pivotal.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
R. Hitt has received honoraria for advisory role from Merck. L. Iglesias has received honoraria for advisory role from Merck, Serono, MSD, BMS, Bayer, Sanofi, AstraZeneca, Novartis and Kura Oncology. A. Berrocal-Jaime has received honoraria for advisory role from BMS, MSD, Roche, Novartis, Pierre Fabre, Merck, Pfizer, Sanofi and Incyte. J. Martínez-Trufero has received honoraria for advisory role from Pharmamar, Merck, Lilly, Eisai, BMS and Bayer. J. Lambrea-Sorrosal has received honoraria for advisory role from Merck, Sanofi, MSD and BMS. E. del Barco-Morillo has received honoraria for advisory role from Roche and AstraZeneca. A. J. Cunquero-Tomas has received honoraria for advisory role from Novartis, Roche, Merck, BMS and Pierre-Fabré. N. Baste has received honoraria for advisory role from Merck, Nanobiotics, AstraZeneca, MSD and BMS. J. J. Cruz has received honoraria for advisory role from Merck, BMS, MSD, Roche, Astra Zeneca and Novartis. All remaining authors have declared no conflicts of interest.
Ethical approval
The study was conducted following the local and national regulations (Spanish agency: Agencia Nacional del Medicamento), including the ethical approval of the ethics committee in each hospital and in compliance with the Helsinki declaration.
Informed consent
All patients received and signed an informed consent following the local and national regulations.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hitt, R., Iglesias, L., López-Pousa, A. et al. Long-term outcomes of induction chemotherapy followed by chemoradiotherapy vs chemoradiotherapy alone as treatment of unresectable head and neck cancer: follow-up of the Spanish Head and Neck Cancer Group (TTCC) 2503 Trial. Clin Transl Oncol 23, 764–772 (2021). https://doi.org/10.1007/s12094-020-02467-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-020-02467-8