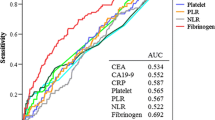
Abstract
Background
We aimed to evaluate the association of preoperative plasma fibrinogen levels with the clinicopathological parameters, disease-free survival, and overall survival in patients with renal cell carcinoma.
Methods
We retrospectively studied 286 patients with renal cell carcinoma who underwent radical nephrectomy from 2000 to 2003 at one center. The plasma fibrinogen was routinely determined before operation in all patients. The correlation of preoperative plasma fibrinogen levels with clinicopathological findings was evaluated by t-test or analysis of variance (ANOVA) methods. As well, univariate and multivariate analyses were used to determine the association between the preoperative level of plasma fibrinogen and survival duration.
Results
An elevated level of plasma fibrinogen was positively related to the Fuhrman grade (P < 0.001), tumor size (P < 0.001), and T stage (P < 0.001), but it was negatively related to histologic type (P = 0.266). Univariate analysis showed that the Fuhrman grade, tumor size, T stage, hemoglobin, corrected calcium, lactate dehydrogenase, and plasma fibrinogen level were significantly correlated with disease-free survival (P < 0.001, P < 0.001, P < 0.001, P < 0.001, P = 0.001, P < 0.001, and P < 0.001, respectively) and overall survival (P < 0.001, P = 0.001, P < 0.001, P < 0.001, P = 0.002, P = 0.001, and P < 0.001). Multivariate analysis showed that the plasma fibrinogen level remained as an independent prognostic factor for disease-free survival (P = 0.021) and overall survival (P < 0.001).
Conclusions
A high preoperative plasma fibrinogen level is an independent predictor of distant metastasis and survival prognosis after radical nephrectomy in patients with renal cell carcinoma.



Similar content being viewed by others
Abbreviations
- RCC:
-
Renal cell carcinoma
- DFS:
-
Disease-free survival
- OS:
-
Overall survival
- CRP:
-
C-reactive protein
- LDH:
-
Lactate dehydrogenase
References
Rickles FR, Edwards RL (1983) Activation of blood coagulation in cancer: Trousseau’s syndrome revisited. Blood 62:14–31
Wang X, Wang E, Kavanagh JJ et al (2005) Ovarian cancer, the coagulation pathway, and inflammation. J Transl Med 3:25
Bardos H, Molnar P, Csecsei G et al (1996) Fibrin deposition in primary and metastatic human brain tumours. Blood Coagul Fibrinolysis 7:536–548
Dvorak HF, Nagy JA, Berse B et al (1992) Vascular permeability factor, fibrin, and the pathogenesis of tumor stroma formation. Ann N Y Acad Sci 667:101–111
Tennent GA, Brennan SO, Stangou AJ et al (2007) Human plasma fibrinogen is synthesized in the liver. Blood 109:1971–1974
Collen D, Tytgat GN, Claeys H et al (1972) Metabolism and distribution of fibrinogen. I. Fibrinogen turnover in physiological conditions in humans. Br J Haematol 22:681–700
Koenig W (2003) Fibrin(ogen) in cardiovascular disease: an update. Thromb Haemost 89:601–609
Altieri DC, Mannucci PM, Capitanio AM (1986) Binding of fibrinogen to human monocytes. J Clin Invest 78:968–976
Dejana E, Languino LR, Polentarutti N et al (1985) Interaction between fibrinogen and cultured endothelial cells. Induction of migration and specific binding. J Clin Invest 75:11–18
Dvorak HF, Harvey VS, Estrella P et al (1987) Fibrin containing gels induce angiogenesis. Implications for tumor stroma generation and wound healing. Lab Invest 57:673–686
Gross TJ, Leavell KJ, Peterson MW (1997) CD11b/CD18 mediates the neutrophil chemotactic activity of fibrin degradation product D domain. Thromb Haemost 77:894–900
Languino LR, Duperray A, Joganic KJ et al (1995) Regulation of leukocyte–endothelium interaction and leukocyte transendothelial migration by intercellular adhesion molecule 1-fibrinogen recognition. Proc Natl Acad Sci USA 92:1505–1509
Robson SC, Saunders R, Purves LR et al (1993) Fibrin and fibrinogen degradation products with an intact D-domain C-terminal gamma chain inhibit an early step in accessory cell-dependent lymphocyte mitogenesis. Blood 81:3006–3014
Gerner C, Steinkellner W, Holzmann K et al (2001) Elevated plasma levels of crosslinked fibrinogen gamma-chain dimer indicate cancer-related fibrin deposition and fibrinolysis. Thromb Haemost 85:494–501
Palumbo JS, Potter JM, Kaplan LS et al (2002) Spontaneous hematogenous and lymphatic metastasis, but not primary tumor growth or angiogenesis, is diminished in fibrinogen-deficient mice. Cancer Res 62:6966–6972
Palumbo JS, Talmage KE, Massari JV et al (2005) Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 105:178–185
Palumbo JS, Kombrinck KW, Drew AF et al (2000) Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood 96:3302–3309
Cavanaugh PG, Sloane BF, Honn KV (1988) Role of the coagulation system in tumor-cell-induced platelet aggregation and metastasis. Haemostasis 18:37–46
Chew EC, Wallace AC (1976) Demonstration of fibrin in early stages of experimental metastases. Cancer Res 36:1904–1909
Crissman JD, Hatfield JS, Menter DG et al (1988) Morphological study of the interaction of intravascular tumor cells with endothelial cells and subendothelial matrix. Cancer Res 48:4065–4072
Hatzfeld JA, Hatzfeld A, Maigne J (1982) Fibrinogen and its fragment D stimulate proliferation of human hemopoietic cells in vitro. Proc Natl Acad Sci USA 79:6280–6284
Pollanen J, Stephens RW, Vaheri A (1991) Directed plasminogen activation at the surface of normal and malignant cells. Adv Cancer Res 57:273–328
Yamashita H, Kitayama J, Kanno N et al (2006) Hyperfibrinogenemia is associated with lymphatic as well as hematogenous metastasis and worse clinical outcome in T2 gastric cancer. BMC Cancer 6:147
Polterauer S, Seebacher V, Hefler-Frischmuth K et al (2009) Fibrinogen plasma levels are an independent prognostic parameter in patients with cervical cancer. Am J Obstet Gynecol 200(647):e641–e647
Polterauer S, Grimm C, Seebacher V et al (2009) Plasma fibrinogen levels and prognosis in patients with ovarian cancer: a multicenter study. Oncologist 14:979–985
Lopez Y, Paloma MJ, Rifon J et al (1999) Measurement of prethrombotic markers in the assessment of acquired hypercoagulable states. Thromb Res 93:71–78
Kerlin B, Cooley BC, Isermann BH et al (2004) Cause-effect relation between hyperfibrinogenemia and vascular disease. Blood 103:1728–1734
Nieswandt B, Hafner M, Echtenacher B et al (1999) Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Cancer Res 59:1295–1300
Yano HJ, Hatano K, Tsuno N et al (2001) Clustered cancer cells show a distinct adhesion behavior from single cell form under physiological shear conditions. J Exp Clin Cancer Res 20:407–412
Yamashita H, Kitayama J, Nagawa H (2005) Hyperfibrinogenemia is a useful predictor for lymphatic metastasis in human gastric cancer. Jpn J Clin Oncol 35:595–600
Acknowledgments
This work was supported by a Grant from the National Natural Science Foundation of China, No. 81072090.
Conflict of interest
We have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
J. Du and J.-H. Zheng contributed equally to this work.
About this article
Cite this article
Du, J., Zheng, JH., Chen, XS. et al. High preoperative plasma fibrinogen is an independent predictor of distant metastasis and poor prognosis in renal cell carcinoma. Int J Clin Oncol 18, 517–523 (2013). https://doi.org/10.1007/s10147-012-0412-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-012-0412-x