Abstract
Due to their specialised habitat requirements, butterflies are particularly vulnerable to habitat loss and fragmentation. Understanding the drivers of local abundances of species is essential for their effective conservation in fragmented landscapes. We investigated factors affecting population densities of an endangered European butterfly, the Violet Copper (Lycaena helle), occurring in a small metapopulation near the city of Kraków, southern Poland. The environmental parameters tested as predictors of the local densities of the species included both the variables associated with spatial structure of habitats such as patch sizes, their isolation and fragmentation as well as those potentially reflecting habitat quality. Patch area and vegetation height turned out to be the only factors significantly influencing L. helle densities, both having a positive effect. The positive impact of patch area is a bit surprising, since its relationship with population densities is typically negative in butterflies. In our study system it is likely to derive from source-sink dynamics as the smaller habitat patches are apparently too small to sustain viable local populations. In turn, the positive influence of vegetation height implies that the ongoing succession does not deteriorate the quality of the recently abandoned meadows yet, whereas higher turf may provide better sheltering places. The loss of almost half of L. helle habitat patches in the study area in recent years is alarming. However, its inclusion into the Natura 2000 system should help to conserve the species as long as this act is followed by proper management of its habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fragmentation and habitat loss are concerned to be among the most serious threats to biodiversity (Saunders et al. 1991; Debinski and Holt 2000; Krauss et al. 2010). Butterflies belong to the organisms that suffer most from these processes, because of their relatively limited dispersal abilities and strong habitat specialization (Thomas and Hanski 1997; van Swaay and Warren 1999; Bergmann et al. 2004), which makes them an useful model group for studies on the effects of habitat loss and fragmentation on species persistence in fragmented landscapes (WallisDeVries et al. 2002). Determining factors which influence patch occupancy and local population densities are key issues in this context. Such factors can generally be divided into two main groups: (1) factors related with habitat quality (e.g., vegetation structure or resource abundance, and (2) factors reflecting spatial structure of habitat patches, mainly their area and connectivity (Thomas et al. 2001; Fleishman et al. 2002). Metapopulation theory focuses primarily on the factors belonging to the latter group. According to the theory, connectivity of habitat patches and their area are crucial predictors for the probability of species occurrence (Hanski 1999). Larger and well-connected patches are less likely to experience extinctions of local population and more likely to become recolonised, and thus more likely to be occupied, which in fact has often been confirmed empirically (Fleishman et al. 2002; Nowicki et al. 2007; Pöyry et al. 2009). In turn, habitat quality defines the carrying capacity of habitat patches and hence it limits local abundances of butterflies (Thomas et al. 2001; Nowicki et al. 2009).
Although the metapopulation theory in its classic form does not consider local population sizes and their densities explicitly, taking these parameters into consideration is of great importance for conservation purposes. Due to stochastic processes smaller populations are more vulnerable to extinctions (Caughley 1994; Schtickzelle et al. 2005). The risk of extinction is further increased through strong Allee effects in small populations (Cantrell and Cosner 2007). Moreover, the number of dispersers and thus the metapopulation persistence is determined not only by local population sizes, but also by their densities through density dependence of emigration rate (Nowicki and Vrabec 2011). Finally, declining population densities can typically be regarded as the first sign of deteriorating habitat quality. Detecting such negative changes and revealing the factors responsible for the process can be very helpful in planning appropriate conservation actions.
The aim of our study was to identify factors affecting local population densities of an endangered butterfly species, the Violet Copper (Lycaena helle). Specifically, we were interested in assessing the relative importance of factors reflecting habitat quality versus those describing spatial structure of habitat patches in shaping local abundances of the species. Due to specialised habitat requirements, the focal species usually forms metapopulations in highly fragmented semi-natural landscapes and thus it appears a good model organism for this type of research. Our ambition was also to use the results obtained for developing practical conservation recommendations.
Materials and methods
Study species
Lycaena helle is a boreo-montane species with the occurrence range extending from Central Europe, Scandinavia and Russia to the Amur Region and Mongolia (Bozano 2004; Habel et al. 2014). In Central Europe it is a postglacial relict, inhabiting mostly highlands (Habel et al. 2011; Martin et al. 2014), although some population occupy wet lowland meadows (Skórka et al. 2007). The species is bivoltine, with adult generations occurring in spring and early summer (Settele et al. 1997; Benes et al. 2002). Its caterpillars are typically monophagous, feeding on the adderwort (Polygonum bistorta) in our study region (Buszko and Maslowski 2008), and thus the occurrence of the species is limited to meadows with this foodplant. Adults can also use buttercups (Ranunculus acer and R. repens) and cuckoo flowers (Cardamine pratensis and C. amara) as nectar sources (Bauerfeind et al. 2009). Lycaena helle is generally regarded as a sedentary species, however it may sporadically perform long-distance movements (Habel et al. 2011). Deterioration of meadow habitats caused by land drainage and abandonment of traditional management, as well as their increasing fragmentation have been suggested as main reasons for the recent decline of the species (Fischer et al. 1999). Lycaena helle is currently one of the most endangered butterfly species in Europe, listed in the European Red Data Book and in the Annexes of the Habitats Directive (Van Helsdingen et al. 1996; van Swaay and Warren 1999).
Study area
The study area was located on the outskirts of Kraków, southern Poland, ca. 5–7 km southwest from the city centre (50°01′N, 19°54′E). It comprises a large wet meadow complex (ca. 800 ha) occupying the flat bed of the Vistula river valley. Its meadow habitats, including wet Molinia meadows (Molinietum caeruleae association) and lowland hay meadows (Arrhenatheretum elatioris association), are mostly abandoned, with a small percentage of the area being mowed annually (Nowicki et al. 2007; see this reference for a detailed description of the study area). Since 2011 a large part of the meadow complex has been protected in the Natura 2000 sites “Dębnicko-Tyniecki Obszar Łąkowy” (PLH 120065) and “Skawiński Obszar Łąkowy” (PLH 120079). Nature 2000 is a pan-European network of protected areas developed for the implementation of the key conservation policies of the European Union, namely its Birds Directive (European Commission 1979) and Habitats Directive (European Commission 1992). Each site in the network is devoted to securing a favourable conservation status of particular species or habitats endangered in Europe (European Commission 2000). Both Natura 2000 sites within our study area have been established for the conservation of four butterfly species of the Annex II of the Habitats Directive: Maculinea teleius, M. nausithous, L. helle, and L. dispar (Kudłek and Pępkowska 2008; Woyciechowski et al. 2008). Prior to the establishment of the Natura 2000 sites urbanisation was a major threat for natural values of the area (cf. Nowicki et al. 2007). Although the meadows fragments vanishing each year due to urban developments were usually small (<1 ha), they often comprised habitats of the aforementioned target species. In addition, some habitat patches of these precious butterflies were lost due to natural succession or ill-advised afforestation projects (Walasz 2008).
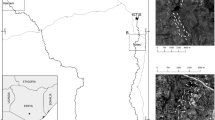
The study area included 21 patches with the P. bistorta foodplant, which can be regarded as the habitat patches of L. helle, when we started the butterfly surveys in 2008 (Fig. 1). Their total area encompassed nearly 23 ha, with individual patch sizes ranging between 0.08 and 2.83 ha (Table 1). The distances between the nearest neighbouring patches were typically 200 to 1500 m. Only 11 patches, though mostly the larger ones, persisted till 2011 (Table 1).
Map of the investigated habitat patches of Lycaena helle, with the inset in the top-left corner showing their approximate location in Poland. Patch numbers correspond to those used in Table 1. The patches that ceased to exist before 2011 are marked with white crosses. Solid lines indicate the boundaries of separate fragments of the two Natura 2000 sites (DTOL = PLH 120065 “Dębnicko-Tyniecki Obszar Łąkowy”; SOL = PLH 120079 “Skawiński Obszar Łąkowy”)
Foodplant and butterfly surveys
We mapped all the P. bistorta patches with ca. 1-m precision using GPS Magellan ProMark X (Magellan System Corp., USA). In order to estimate the foodplant density we counted its shoots within 3–5 randomly selected 5 × 5 m plots at each patch in June 2008 and 2011. The exact number of shoots was recorded except for a few cases in 2008, in which it was estimated with classes of ten or multiples of ten. In those cases we adopted the mid-class value when calculating the foodplant density as the mean value for both years of the study.
Butterflies surveys were conducted for the summer generation in 2008 and the spring generation in 2011, using tested and parameterized catch per-unit-time method (Nowicki et al. 2007; Niedobecki 2011). Surveys involved monitoring adult abundance and sex ratios at two representative patches through capturing individuals during 1-h capture sessions conducted in fine weather between 9:00 and 17:00. The capture sessions were held daily to every second day throughout the entire flight period (27 June to 3 August 2008, and 8 May to 9 June 2011). Peak occurrence periods of adults in both seasons (summer 2008 and spring 2011) were determined on the basis of the highest daily numbers of captured individuals and their balanced sex ratio, i.e., approximately 60 % proportion of males concerning their higher catchability (Nowicki et al. 2005a). Subsequently, during these peak adult occurrence periods we conducted 1-h capture sessions at each habitat patch for 2 days (8–9 July 2008, as well as 24 May and 26 May 2011). While the data collected in this way were not comprehensive enough to yield seasonal population sizes with mark-capture models, they allowed the estimation of peak population sizes based on capture frequencies (cf. Nowicki et al. 2007). Peak population size estimates were subsequently extrapolated into seasonal population size estimates following the approach described by Nowicki et al. (2005a), which accounts for flight period length and average adult life span. The latter was calculated as \(e = (1 - \phi )^{ - 1} - 0.5\) (Nowicki et al. 2005b), where Φ represents mean daily survival rate derived with the Cormack–Jolly–Seber mark-recapture model (Schwarz and Arnason 1996; Schwarz and Seber 1999) using the MARK 5.1 software (White and Burnham 1999). The adult life span estimates reached 4.99 and 5.93 days in summer 2008 and spring 2011 respectively.
Statistical analysis
The population size estimates were converted into seasonal densities through division by patch area. In several temperate zone butterflies spring generations were found to be several times smaller than summer ones (Fric et al. 2006; Pickens 2007), which was also the case in our study on L. helle. Thus, in order to make possible the comparison of seasonal densities recorded at particular patches in summer 2008 and spring 2011, we used their standardised values. Standardisation was done through dividing densities recorded at each patch by mean density across all the patches in a given season. Subsequently, for each patch we calculated the mean of the standardised density values obtained for summer 2008 and spring 2011. In this way we derived relative density index, which was used as the dependent variable in the analysis of factors affecting local abundances of L. helle. For the patches that ceased to exist before 2011, i.e., in the cases with no standardised density value available for spring 2011, the standardised density values obtained for summer 2008 were adopted as the relative density index.
We opted for deriving a combined density index for both investigated seasons instead of analysing the patterns in local abundances of the focal species separately for summer 2008 and spring 2011, because the population sizes recorded at each habitat patch within a 3-year year period are likely to be non-independent. In other words, we wanted to avoid the problem of temporal autocorrelation in local population densities, which should be expected within such a short period (cf. Royama 1992; Liebhold et al. 2004), even though it could not be assessed with the data from just two seasons. In turn, it must be underlined that there was absolutely no indication of spatial autocorrelation in local abundances of L. helle across the investigated patches. Mantel test r values were very close to zero for population densities recorded in both seasons (summer 2008: r = 0.0729, P = 0.2035; spring 2011: r = −0.0439, P = 0.3471) as well as for the combined density index (r = 0.0631, P = 0.2557).
The factors tested as predictors of local abundances of L. helle included both the parameters associated with spatial structure of habitats such as patch sizes, their isolation and fragmentation as well as those potentially reflecting habitat quality, including anthropogenic pressure (see Table 2 for details). Except for the foodplant density and vegetation height, the predictor values were derived from the existing GIS database of the study area, using the Idrisi 2.0 software (Eastman 1997). Foodplant density and distance to streams were logarithmically transformed in order to achieve their normal distributions. When estimating the connectivity of each habitat patch, which depends on the distances to all other patches in the system (Hanski et al. 1994), we used the spatial configuration of the patches existing in summer 2008. However, with this parameter based on the patch configuration in spring 2011, which we also attempted for comparison, the outcome of all the analyses remained virtually unchanged.
All the parameters used as predictors were only moderately correlated with one another (Pearson’s r invariably below 0.5, thus within the range in which variables in multivariate analyses may be regarded as fairly independent; Legendre and Legendre 1998). Their effect on local abundances of L. helle was analysed with multiple linear regression model with a forward stepwise variable selection. Nevertheless, it should be stressed that a backward elimination procedure would not make any difference as it yielded identical final results. The statistical analyses were performed using the Statistica 10 program (Statsoft 2010), except for the Mantel tests, which were conducted with the help of the program Zt (Bonnet and Van de Peer 2002).
Results
In summer 2008 L. helle was found in 19 out 21 habitat patches, while in spring 2011 it occupied the 11 remaining patches (Table 1). During the capture sessions we recorded 938 adults in summer 2008 and 241 adults in spring 2011. The total metapopulation size was estimated at 5367 adult butterflies for the summer 2008 generation, with ten relatively large local populations (>100 adults) encompassing together 93 % of all individuals. Seven of these large populations persisted till spring 2011, when they jointly constituted 90 % of the total metapopulation estimated at 1271 individuals. Butterfly densities at particular patches in summer 2008 varied greatly between 95 and 1259 individuals per ha (coefficient of variation CV = 0.91). In spring 2011 they were much more uniform, ranging between 67 and 119 individuals per ha (CV = 0.19).
The multiple regression analyses, using both stepwise and backward variable selection, indicated that local densities of L. helle were significantly influenced by patch area and vegetation height, and the density index positively correlated with both above variables (Table 3). The results of the stepwise variable selection procedure showed that patch area explained 24.4 % of the variation in the density index, and vegetation height explained further 20.2 % (Table 3). The overall model fit was thus moderately good, reaching 44.6 %.
Discussion
The outcome of our study allows a reliable assessment of the status of L. helle metapopulation within the study area. From the conservation point of view our results are of serious concern. Obviously, the fact that the metapopulation size estimated in summer 2008 was approximately four times larger than the one in spring 2011 should not necessarily be perceived as an indication of a sharp decline in L. helle abundance within the investigated period. Instead, it is likely to reflect the difference between summer and spring generations, as the former ones are typically several times larger in bivoltine butterflies of the temperate zone (Fric et al. 2006; Pickens 2007). However, disappearing of more than 40 % of occupied patches within the 3 years of our study, even though they were generally of low quality and inhabited by small populations, is alarming. These ‘lost’ patches encompassed 36 % of area occupied by L. helle and 17.5 % of its seasonal numbers in 2008.
The fact that patch area explained a considerable fraction of variation in L. helle densities across the habitat patches seems to be a bit surprising, mainly because of the positive relationship. While patch area is a good predictor of both the probability of species occurrence and population size (Hanski et al. 1995; Hill et al. 1996; Bauerfeind et al. 2009), it rarely correlates with population density. Moreover, the result of the meta-analysis by Hambäck and Englund (2005) suggests that whenever such a correlations exists in butterflies it is generally negative. A possible explanation for the positive correlation between patch area and L. helle densities that we recorded may be a situation, in which L. helle in our study area does not exist in a metapopulation structure but rather in a source-sink system (sensu Pulliam and Danielson 1991).
Obviously, the data we have gathered do not allow proving the existence of such a system, as it would require profound information about population dynamics within habitat patches and dispersal among them. Nevertheless, some indirect evidence makes plausible our hypothesis concerning possible source-sink dynamics in the investigated system. First of all, the small habitat patches may actually be too small to sustain L. helle populations, and thus individuals observed at such patches may mostly represent immigrants from larger patches (cf. Brückmann et al. 2010). This postulate is supported by the fact that spring population sizes in several small patches reached only ca. 30–40 individuals. Due to temporal fragmentation of the populations, i.e., the fact that only a fraction of individual is present at any day of the season as their life span is much shorter than flight period length (Nowicki et al. 2005b), such low numbers make finding a mating partner difficult (and sometimes even impossible), thus negatively affecting population growth.
The positive relationship between patch area and L. helle densities is also likely to be supported by asymmetric dispersal. A positive effect of patch area on immigration probability and its negative effect on emigration rate have often been reported for butterflies (Wahlberg et al. 2002; Rabasa et al. 2008; Bonelli et al. 2013). Consequently, large patches should mostly gain dispersing individuals, whereas the small ones should mostly loose them. However, our argument that the observed positive density-area relationship may derive from asymmetric dispersal can be valid only if there is considerable dispersal among the local populations. Such an assumption seems to contradict the strong site-fidelity reported for L. helle (Habel et al. 2011). However, our anecdotal observations of inter-patch movements over a few hundred meter distances (Nabielec and Nowicki, unpublished data) as well as the results of a recent quantitative analysis of the species mobility (Craioveanu et al. 2014) indicate that L. helle may be more mobile than it is popularly believed.
A positive correlation between meadow vegetation height and L. helle densities was also detected. Negative relationship would indicate that succession may threaten the persistence of L. helle populations. Nevertheless, an earlier study in the same region showed that in its initial stages the succession at recently abandoned wet meadows improves habitat quality for many butterflies, although in later stages its effect is definitely detrimental (Skórka et al. 2007). Additionally, higher vegetation can act as shelter from bad weather conditions e.g., strong wind (Dennis 2004), and specifically in L. helle it can be used for roosting at night or in poor weather (Turlure et al. 2009; Goffart et al. 2014).
The lack of the effect of foodplant availability on L. helle densities may seem a bit surprising, but it most likely reflects the fact that P. bistorta is overabundant at the investigated patches (reaching 3–263 shoots per m2) and it does not limit the butterfly populations. The species densities were also not influenced by patch connectivity, but again connectivity does not appear to a limiting factor in our study system, because most of the habitat patches (especially the centrally located large ones) are rather well-connected. However, it must be noted that studies focusing on L. helle patch occupancy indicated positive effects of patch sizes and their connectivity (Fischer et al. 1999; Bauerfeind et al. 2009).
To conclude, local densities of L. helle proved to be influenced by both spatial structure of habitat patches, as indicated by a significant effect of patch area, as well as by habitat quality, represented by vegetation height. Such an outcome is in contrast with the findings of our earlier study on the abundance patterns on Maculinea butterflies, for which only spatial structure of habitat patches turned out to matter (Nowicki et al. 2007). The implication is that in order to support the persistence of L. helle within the investigated region the conservation efforts are needed both at landscape scale, so as to ensure the existence of a number of large habitat patches, as well as at within-patch scale, where they should be focused on improving habitat quality.
The inclusion of the majority of studied area into the Natura 2000 network offers a chance for improving the status of L. helle in the region. Thus, from the conservation point of view, the priority should be preserving the group of large and well-connected patches of P. bistorta in the central part of the meadow complex (see Fig. 1). The remaining patch (number 10 in Fig. 1), although relatively large as well, is probably too isolated to effectively serve as a source of immigrants to other populations. According to Hanski et al. (1996) a stable metapopulation should ideally consist of at least 15 well-connected populations, while the aforementioned key group of patches hosts only ten populations. Therefore it is vital that every single of them is preserved, including the two (patches 12 and 13 in Fig. 1), which are located directly outside the boundary of the Natura 2000 site “Dębnicko-Tyniecki Obszar Łąkowy”. This should serve as a strong argument for extending this Natura 2000 site so as to include the focal meadow fragment, which in fact was the case in the initial proposal for the site (Kudłek and Pępkowska 2008).
Obviously, the inclusion into the Natura 2000 network will help L. helle population only if it is followed by proper management of their patches. Rotational mowing and extensive grazing are believed to be the most effective management methods for L. helle habitats (Fischer et al. 1999; Bauerfeind et al. 2009). Incorporating such management into action plans for the meadows occupied by L. helle will surely contribute to its more effective conservation. Moreover, these types of land management are also favourable for many other butterfly species (Elligsen et al. 1997; Meyer-Hozak 2000; Dover et al. 2011).
References
Bauerfeind SS, Theisen A, Fischer K (2009) Patch occupancy in the endangered butterfly Lycaena helle in fragmented landscape: effects of habitat quality, patch size and isolation. J Insect Conserv 13:271–277
Benes J, Konvicka M, Dvorak J, Fric Z, Havelda Z, Pavlicko A, Vrabec V, Weidenhoffer Z (2002) Butterflies of the Czech Republic: distribution and conservation I. II, SOM, Praha
Bergmann KO, Askling J, Ekberg O, Ignell H, Wahlman H, Milberg P (2004) Landscape effects on butterfly assemblages in an agricultural region. Ecography 27:619–628
Bonelli S, Vrabec V, Witek M, Barbero F, Patricelli D, Nowicki P (2013) Selection on dispersal in isolated butterfly metapopulations. Popul Ecol 55:469–478
Bonnet E, Van de Peer Y (2002) Zt: a software tool for simple and partial Mantel tests. J Stat Softw 7:1–12
Bozano GC (2004) Guide to the butterflies of the Palaearctic Region: Lycaenidae. Omnes Artes, Milano
Brückmann SV, Krauss J, Steffan-Dewenter I (2010) Butterfly and plant specialists suffer from reduced connectivity in fragmented landscapes. J Appl Ecol 47:799–809
Buszko J, Maslowski J (2008) Motyle dzienne Polski. Koliber, Nowy Sacz (in Polish)
Cantrell RS, Cosner C (2007) Density dependent behavior at habitat boundaries and the Allee effect. Bull Math Biol 69:2339–2360
Caughley G (1994) Directions in conservation biology. J Anim Ecol 63:215–244
Craioveanu C, Sitar C, Rakosy L (2014) Mobility, behaviour and phenology of the Violet Copper Lycaena helle in North-Western Romania. In: Habel JC, Meyer M, Schmitt T (eds) Jewels in the mist. A synopsis on the endangered Violet Copper butterfly Lycaena helle. Pensoft, Sofia-Moscow, pp 91–105
Debinski DM, Holt RD (2000) A survey and overview of habitat fragmentation experiments. Conserv Biol 14:342–355
Dennis RLH (2004) Just how important are structural elements as habitat components? Indications from a declining lycaenid butterfly with priority conservation status. J Insect Conserv 8:37–45
Dover JW, Rescia A, Fungariño S, Fairburn J, Carey P, Lunt P, Arnot C, Dennis RLH, Dover CJ (2011) Land-use, environment, and their impact on butterfly populations in a mountainous pastoral landscape: species richness and family-level abundance. J Insect Conserv 15:523–538
Eastman JR (1997) Idrisi for Windows. User’s Guide Version 2.0. Clark University, Worchester, MA, USA
Elligsen H, Beinlich B, Plachter H (1997) Effects of large scale cattle grazing on populations of Coenonympha glycerion and Lasiommata megera (Lepidoptera: Satyridae). J Insect Conserv 1:13–23
European Commission (1979) Council Directive 79/409/EEC of April 1979 on the conservation of wild birds. Off J Eur Union Legis 103:1
European Commission (1992) Council Directive 92/43/EEC of 21 May 1992 on the Conservation of natural habitats and of wild fauna and flora. Off J Eur Union Legis 206:7
European Commission (2000) Natura 2000. Managing our heritage, EU, Luxembourg
Fischer K, Beinlich B, Plachter H (1999) Population structure, mobility and habitat preferences of the violet copper Lycaena helle (Lepidoptera: Lyceanidae) in Western Germany: implications for conservation. J Insect Conserv 3:43–52
Fleishman E, Ray C, Sjögren-Gulve P, Boggs CL, Murphy DD (2002) Assessing the roles of patch quality, area, and isolation in predicting metapopulation dynamics. Conserv Biol 16:706–716
Fric Z, Klimova M, Konvicka M (2006) Mechanical design indicates differences in mobility among butterfly generations. Evol Ecol Res 8:1511–1522
Goffart P, Cavelier E, Lighezzolo P, Rauw A, Lafontaine D (2014) Restoration and management of habitat networks for Lycaena helle in Belgium. In: Habel JC, Meyer M, Schmitt T (eds) Jewels in the mist. A synopsis on the endangered Violet Copper butterfly Lycaena helle. Pensoft, Sofia-Moscow, pp 197–216
Habel JC, Rodder D, Schmitt T, Néves G (2011) Global warming will affect the genetic diversity and uniqueness of Lycaena helle populations. Global Change Biol 17:194–205
Habel JC, Meyer M, Schmitt T (2014) Jewels in the mist. A synopsis on the endangered Violet Copper butterfly Lycaena helle. Pensoft, Sofia-Moscow
Hambäck PA, Englund G (2005) Patch area, population density and the scaling of migration rates: the resource concentration hypothesis revisited. Ecol Lett 8:1057–1065
Hanski I (1999) Metapopulation ecology. Oxford University Press, Oxford
Hanski I, Kuussaari M, Nieminen M (1994) Metapopulation structure and migration in the butterfly Melitaea cinxia. Ecology 75:747–762
Hanski I, Pakkala T, Kuussari M, Lei G (1995) Metapopulation persistence of an endangered butterfly in a fragmented landscape. Oikos 72:21–28
Hanski I, Moilanen A, Gyllenberg M (1996) Minimum viable metapopulation size. Am Nat 147:527–541
Hill JK, Thomas CD, Lewis OT (1996) Effects of habitat patch size and isolation on dispersal by Hesperia comma butterflies: implications for metapopulation structure. J Anim Ecol 65:725–735
Krauss J, Bommarco R, Guardiola M, Heikkinen RK, Helm A, Kuussaari M, Lindborg R, Öckinger E, Pärtel M, Pino J, Pöyry J, Raatikainen KM, Sang A, Stefanescu C, Teder T, Zobel M, Steffan-Dewenter I (2010) Habitat fragmentation causes immediate and time-delayed biodiversity loss at different trophic levels. Ecol Lett 13:597–605
Kudłek J, Pępkowska A (2008) Natura 2000 Standard Data Form for SCI Dębnicko-Tyniecki Obszar Łąkowy PLH 120065. GDOŚ, Kraków (in Polish)
Legendre P, Legendre L (1998) Numerical ecology. Elsevier, Amsterdam
Liebhold A, Koenig WD, Bjørnstad ON (2004) Spatial synchrony in population dynamics. Annu Rev Ecol Evol Syst 35:467–490
Martin Y, Habel JC, Van Dyck H, Titeux N (2014) Losing genetic uniqueness under global change: the Violet Copper (Lycaena helle) in Europe. In: Habel JC, Meyer M, Schmitt T (eds) Jewels in the mist. A synopsis on the endangered Violet Copper butterfly Lycaena helle. Pensoft, Sofia-Moscow, pp 165–184
Meyer-Hozak C (2000) Population biology of Maculinea rebeli (Lepidoptera: Lycaenidae) on the chalk grasslands of Eastern Westphalia (Germany) and implications for conservation. J Insect Conserv 4:63–72
Niedobecki D (2011) Valorisation of butterfly communities within the area of the planned Natura 2000 sites “Łąki Nowohuckie” oraz “Łąki nad Rudawą”. MSc thesis, Uniwersytet Jagiellonski, Kraków (in Polish with English abstract)
Nowicki P, Vrabec V (2011) Evidence for positive density-dependent emigration in butterfly metapopulations. Oecologia 167:657–665
Nowicki P, Richter A, Glinka U, Holzschuh A, Toelke U, Henle K, Woyciechowski M, Settele J (2005a) Less input same output—simplified approach for population size assessment in Lepidoptera. Popul Ecol 47:203–212
Nowicki P, Witek M, Skórka P, Settele J, Woyciechowski M (2005b) Population ecology of the endangered butterflies Maculinea teleius and M. nausithous and the implications for conservation. Popul Ecol 47:193–202
Nowicki P, Pępkowska A, Kudlek J, Skórka P, Witek M, Settele J, Woyciechowski M (2007) From metapopulation theory to conservation recommendations: lessons from spatial occurrence and abundance patterns of Maculinea butterflies. Biol Conserv 140:119–129
Nowicki P, Bonelli S, Barbero F, Balletto E (2009) Relative importance of density-dependent regulation and environmental stochasticity for butterfly population dynamics. Oecologia 161:227–239
Pickens BA (2007) Understanding the population dynamics of a rare, polyvoltine butterfly. J Zool 273:229–236
Pöyry J, Paukkunen J, Heliölä J, Kuussaari M (2009) Relative contributions of local and regional factors to species richness and total density of butterflies and moths in semi-natural grasslands. Oecologia 160:577–587
Pulliam HR, Danielson BJ (1991) Sources, sinks, and habitat selection: a landscape perspective on population dynamics. Am Nat 137:50–66
Rabasa SG, Gutiérrez D, Escudero A (2008) Relative importance of host plant patch geometry and habitat quality on the patterns of occupancy, extinction and density of the monophagous butterfly Iolana iolas. Oecologia 156:491–503
Royama T (1992) Analytical population dynamics. Chapman & Hall, London
Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation—a review. Conserv Biol 5:18–32
Schtickzelle N, Choutt J, Goffart P, Fichefet V, Baguette M (2005) Metapopulation dynamics and conservation of the marsh fritillary butterfly: population viability analysis and management options for a critically endangered species in Western Europe. Biol Conserv 126:569–581
Schwarz CJ, Arnason AN (1996) A general methodology for the analysis of capture-recapture experiments in open populations. Biometrics 52:860–873
Schwarz CJ, Seber GAF (1999) Estimating animal abundance: review III. Stat Sci 14:427–456
Settele J, Feldmann R, Reinhardt R (eds) (1997) Die Tagfalter Deutschlands. Ulmer, Stuttgart (in German)
Skórka P, Settele J, Woyciechowski M (2007) Effects of management cessation on grassland butterflies in southern Poland. Agric Ecosyst Environ 121:319–324
StatSoft Inc. (2010) Statistica for Windows. Version 10. Tulsa, USA
Thomas CD, Hanski I (1997) Butterfly metapopulations. In: Hanski I, Gilpin ME (eds) Metapopulation biology. Ecology, genetics, and evolution. Academic Press, San Diego, CA, pp 359–386
Thomas JA, Bourn NAD, Clarke RT, Stewart KE, Simcox DJ, Pearman GS, Curtis R, Goodger B (2001) The quality and isolation of habitat patches both determine where butterflies persist in fragmented landscapes. Proc R Soc B 268:1791–1796
Turlure C, Van Dyck H, Schtickzelle N, Baguette M (2009) Resource-based habitat definition, niche overlap and conservation of two sympatric glacial relict butterflies. Oikos 118:950–960
Van Helsdingen PJ, Willemse L, Speight MCD (1996) Background information on invertebrates of the Habitats Directive and the Bern Convention. Part I–Crustacea, Coleoptera and Lepidoptera. Nature and Environment No. 79. Council of Europe Publishing, Strasbourg
van Swaay CAM, Warren MS, (1999) Red Data Book of European Butterflies (Rhopalocera), Nature and Environment No. 99. Council of Europe Publishing, Strasbourg
Wahlberg N, Klemetti T, Selonen V, Hanski I (2002) Metapopulation structure and movements in five species of checkerspot butterflies. Oecologia 130:33–43
Walasz K (ed) (2008) Inwentaryzacja i waloryzacja Dębnicko-Tynieckiego Obszaru Łąkowego zgłoszonego do ochrony jako obszar Natura 2000 ze szczególnym uwzględnieniem terenu Zakrzówka. Uniwersytet Jagiellonski, Kraków (in Polish)
WallisDeVries MF, Poschlod P, Willems JH (2002) Challenges for the conservation of calcareous grasslands in Northwestern Europe: integrating the requirements of flora and fauna. Biol Conserv 104:265–273
White G, Burnham K (1999) Program MARK: survival estimation from populations of marked animals. Bird Study 46:120–138
Woyciechowski M, Nowicki P, Kudłek J, Pępkowska A (2008) Natura 2000 standard data form for Obszaru SCI Skawiński Obszar Łąkowy PLH 120079. GDOŚ, Kraków (in Polish)
Acknowledgments
The study was funded by the Polish National Science Centre Grant DEC-2013/11/B/NZ8/00912 and by the Jagiellonian University through its DS/WBINOZ/INOŚ/761/09-10 funds. We are also grateful to Wojciech Tokarz for his help in the fieldwork, and to Christine Richards for improving the English of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nabielec, J., Nowicki, P. Drivers of local densities of endangered Lycaena helle butterflies in a fragmented landscape. Popul Ecol 57, 649–656 (2015). https://doi.org/10.1007/s10144-015-0507-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-015-0507-0