Abstract
Today, many seabird species nest in port areas, which are also necessary for human economic activity. In this paper, we evaluate, using a metapopulation model, the possibilities for creating alternative breeding sites for the Common Tern (Sterna hirundo) in the Rhine–Meuse–Scheldt estuary. We explore 22 scenarios that differ with respect to (1) loss of breeding habitat in port areas, (2) location and size of newly created habitat, and (3) coexistence of old and new habitat. Results indicate that loss of port area habitats results in a serious 41% decline in the breeding population. When the loss in ports is compensated for within the ports, the decline was negligible. Fourteen scenarios result in an increase of the Common Tern metapopulation. In these, extra breeding habitat is created outside the ports in fish-rich waters, resulting in a potential metapopulation increase of 25%. However, the period of overlap between lost and newly created habitat strongly affects the results. A gap between the removal of old and the creation of new breeding areas might cause a drop in the metapopulation level of 30%. The population recovery from this drop might take more than 100 years due to slow recolonization. Our results suggest that conservation of seabird species should be evaluated on a metapopulation scale and that the creation of new habitat may help to compensate for habitat loss in other areas. Furthermore, the results indicate that overlap between the existence of old and newly created breeding habitats is crucial for the success of compensation efforts. However, new locations should be carefully selected, because not only is the suitability of the breeding grounds important, but ample fish availability nearby is also key.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coastal areas and estuaries offer important habitat for many seabird species (Ysebaert et al. 2000). In recent decades, however, human activities have affected the natural habitats in many estuaries. Dykes have been built to reclaim land from the sea, cities and ports have been developed, sea arms have been embanked, and beaches and dunes occupied by recreants. As a result, coastal birds that depend on temporary natural islands and sandbanks and shell deposits for their breeding colonies now suffer a lack of natural breeding grounds.
Harbours and ports typically incorporate newly developed terrains that offer temporarily suitable areas for coastal birds to breed. These locations are relatively undisturbed by recreants and not yet colonized by ground predators. That coastal birds are increasingly colonizing these areas is a good thing (Stienen et al. 2005), but when these terrains are needed for economic purposes the birds’ presence becomes inconvenient, since many coastal breeders are endangered and protected by law. This has led to conflicts among stakeholders, especially between environmental associations and the authorities in charge of port maintenance and improvement. This problem might be solved with the creation of alternative safe habitats in less economically important areas or on new artificially created islands (Erwin et al. 1995, 1998).
Seabird population dynamics are characterized by high adult longevity and low recruitment (Martinez-Abrain et al. 2003; Cam et al. 2004; Oro et al. 2004; Becker and Bredley 2007). An adult Common Tern (Sterna hirundo), for example, lives about 10 years whereas recruitment per year is only 0.28 juveniles per female (Schroder et al. 1996). The breeding phase is the sensitive period, because it is stressful for adults energy-wise and because eggs and young birds are vulnerable to predation (Akçakaya et al. 2003; Stienen and Brenninkmeijer 2006; Jones et al. 2008). Seabirds prefer nesting on islands that are regularly flooded. Here, predator numbers are limited and vegetation is kept at the pioneer stage, which enables adults to spot potential dangers more readily. Yet another important factor that determines breeding success is availability of food in the surrounding waters (Stienen et al. 2000; Stienen and Brenninkmeijer 2002; Oro 2003). Thus, both nest safety and fish availability largely determine a colony’s success.
Because seabirds are site-faithful and tend to return to their previously used nesting area (Spendelow et al. 1995; Van der Hoorn et al. 1997), we can link birds to a specific breeding patch. The few birds that do not return to their original breeding site, nesting instead at an alternative location, can be regarded as dispersing animals. A number of connected breeding sites is called a metapopulation (Spendelow et al. 1995; Akçakaya et al. 2003; Oro 2003; Serrano and Tella 2003). Opdam (1991) described metapopulations as spatially structured populations of plants or animals consisting of distinct units (subpopulations), separated by space or barriers and connected via dispersal movements. The spatial distribution of habitat patches and the connectivity between these patches largely determine survival and size of the individual populations (Schippers et al. 1996; Vermaat et al. 2008). In contrast to other metapopulation studies (Verboom et al. 1991; Van Apeldoorn et al. 1998; Alonso et al. 2004; Morales et al. 2005), in the case of the Common Tern the size of the breeding habitat is not directly related to the carrying capacity of an individual population, since food abundance in the surrounding waters also determines a large part of breeding success.
Metapopulations characteristically demonstrate a turnover, with populations going extinct in some localities and other sites being recolonized, resulting in a distribution pattern that shifts over time (Levins 1970; Hanski and Gilpin 1991; Opdam 1991; Sachot et al. 2006). In the past decade, metapopulation models have been developed for many species to evaluate the relation between habitat changes and species responses. The current study applies such a model to investigate the possibilities for creating alternative patches to compensate for potential habitat loss for the Common Tern (Sterna hirundo) in the Rhine–Meuse–Scheldt estuary. We use this model to answer two main questions:
-
What are the consequences of habitat loss in, for example, port areas for seabird populations?
-
Can newly created breeding habitat for seabirds replace lost habitats in industrial areas?
Our study focuses on the metapopulation dynamics of the Common Tern in the Dutch–Belgian coastal region. This is an area of international importance for seabird conservation (Ysebaert et al. 2000) yet which also accommodates two of the world’s largest ports. We selected the Common Tern because it is an opportunistic species that frequently settles in man-made sites (Meininger et al. 2000; Becker and Ludwigs 2004; Strucker et al. 2005; Courtens et al. 2007). Furthermore, the literature provides local data on life history for this species (Stienen and Brenninkmeijer 1992; Schroder et al. 1996; Van der Hoorn et al. 1997; Becker and Ludwigs 2004). We are particularly interested in the role that port habitats play in the survival of coastal birds, as in recent years these sites have become increasingly popular as breeding grounds for terns, gulls and other seabirds.
Materials and methods
Study area
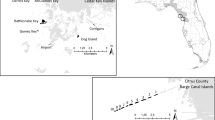
Our study area is the Rhine–Meuse–Scheldt ‘delta’. For a few hundred years, this delta (in fact an estuary) consisted of a large number of islands divided by dynamic branches of sea. In the course of the past three to four centuries most of these islands became interconnected by polders, and these larger semi-islands were connected by dams and bridges. Nowadays, the area acts as a gateway to Europe with the international ports of Rotterdam and Antwerp and the smaller port of Zeebrugge located there. Parts of the delta are no longer influenced by tide and have been converted to stagnant salt water or freshwater systems. All the major Common Tern colonies observed in recent years are found in the vicinity of the salt water (Fig. 1). The estuaries and port areas act as a continuous dynamic habitat matrix, with large fluctuations at the local level but a relatively stable metapopulation on a regional (delta) scale, at least during the past 15 years. Apart from the intensive exchange of individuals among the delta habitats, there is also a small exchange of individuals with coastal bird populations in the Wadden Sea and IJsselmeer area and in the coastal regions of the United Kingdom. Because this exchange is relatively small (Stienen and Brenninkmeijer 1992; Schroder et al. 1996), we assume that the net colonization from other metapopulations equals the net dispersal to these populations.
Common Tern data analysis
Data on the distribution of the Common Tern in the study area were provided by the Dutch National Institute for Sea and Marine Management (RIKZ) and the Belgian Research Institute for Nature and Forest (INBO). Breeding pair numbers were given per location for the period 1991–2005 (Netherlands) and 1960–2005 (Belgium). These data provide insights into population trends in the past decades, as well as estimates of the carrying capacity of the different breeding locations. We used the maximum number of pairs observed at each of the 45 selected locations as an indication of the carrying capacity of these locations. Furthermore, data were used to indicate the presence or absence of birds at each location and the initial number of birds that first colonized the location. Table 1 lists this data by location.
The Common Tern breeds at sites located close to fish-rich waters (maximum distance 3–10 km), where they forage food to raise their young (Becker et al. 1993; Becker and Ludwigs 2004). The sites usually have bare ground or short grass, giving the birds an overview of approaching dangers (Becker and Ludwigs 2004). We derived a habitat patch map from the Common Tern census data, assuming that each location where the bird species was observed breeding in recent years can be regarded as a habitat patch. For simplicity, we used only the 45 largest bird breeding locations, which together accommodated more than 95% of the Dutch–Belgian delta metapopulation, calculated over 1991–2005 (Fig. 1; Table 1). Furthermore, we analyzed the data to obtain a picture of the breeding pair variability at the individual sites, the total metapopulation trend and the fraction of the metapopulation nesting in port areas.
Modeling the metapopulation dynamics of the Common Tern
We use the metapopulation model “METAPOP” (Van Apeldoorn et al. 1998; Verboom et al. 2001; Vos et al. 2001) to simulate the dynamics of the Common Tern in the Dutch–Belgian delta region. Our model calculates the effects of changes in the configuration of habitat patches induced by habitat redesign. Our model is spatially explicit simulating Common Tern females in space and time. The model can be used to simulate population dynamics or to estimate survival probabilities. The life history events for each individual are reproduction, dispersal, aging and mortality. Additionally, habitat dynamics describe the probability that a suitable habitat will become unsuitable and vice versa, taking into account vegetation development and flooding of breeding areas. The life history events occur sequentially during the year:
-
1.
Reproduction: each adult female has the probability of producing one-year-old recruits according to a Poisson distribution.
-
2.
Dispersal: the dispersal process is divided into three parts:
-
(a)
Probability to disperse, which is the likelihood that an individual bird in a colony will disperse.
-
(b)
Probability to arrive at another patch, determined by the carrying capacity and distance from the original site (the larger the carrying capacity of a patch and the closer the proximity, the higher the probability to arrive).
-
(c)
Probability that a dispersing bird will indeed settle at the patch where it has arrived, which is dependent on local bird numbers and relative density.
-
(a)
-
3.
Survival (mortality): the probability of a bird surviving from one year to the next.
-
4.
Aging: the probability that a juvenile will grow into adulthood. This is age dependent.
-
5.
Habitat dynamics: The probability that a suitable habitat patch will become unsuitable and vice versa.
We make both reproduction and dispersal density dependent. The metapopulation model requires a number of parameters on various aspects of the life history of the species. S1 in Electronic Supplementary Material (ESM) provides more detail on the life history modeling of the Common Tern.
Scenarios
We created four main scenarios that differ with respect to loss of breeding habitat in port areas and the location and size of newly created habitat. We selected seven breeding locations in port areas to explore the role of ports in providing breeding habitat for the Common Tern (Table 2). These locations represent areas of major economic activity in the port as well as key breeding locations for the Common Tern. In the main scenarios, we varied only the carrying capacity and location of the seven selected port locations (Table 2). The conditions at the other 38 locations were the same for all scenarios.
Scenario 1: no sites in ports
In this scenario, we started the simulation with the present situation according to Table 1. After 50 years, all habitats in the ports are lost (see zero values in Table 2). The results give us an appreciation of the importance of the port locations for the network population.
Scenario 2: compensation in ports
In the second scenario, we replaced the lost habitats primarily within the ports themselves, usually within 2 km of the original location (Table 2). Since birds would still then be foraging in the same waters, the carrying capacity of these in-port locations was kept the same as in the original port habitats. In the case of Zeebrugge, the replacement of the ‘Tern Peninsula’ (Sternenschiereiland) habitat was divided over two locations, together having a similar carrying capacity to that of Tern Peninsula in the present situation.
Scenario 3: compensation near ports
The vicinity of ports may provide better locations for breeding sites than the ports themselves, as nearby spaces are available that can accommodate larger and safer breeding sites, and locations can be selected nearer fish-rich feeding grounds. To account for these advantages, we enlarged the carrying capacity of the replacement sites in the vicinity of the ports compared to present conditions. Carrying capacity was estimated based on the more suitable locations currently found outside the ports for the Common Tern. Compensatory locations were mostly within 5 km of the original location. The Oostvoorne–Europoort population was assumed to be compensated by both other Oostvoorne populations.
Scenario 4: compensation in central locations
For the last scenario, we located replacement sites for all port habitats towards the centre of the study area (Fig. 1, arrows), as this would theoretically be the best option to strengthen metapopulations (Pulliam 1988; Wiens 1989). The distance from the original populations varied between 4 and 66 km. So, whereas the carrying capacity of the sites is the same as in scenario 3, the replacement sites were moved away from the port area to suitable places specifically at the heart of the Common Tern metapopulation in the Dutch part of the delta region. This enabled us to explore the impact of the location of the replacement site. Also as in scenario 3, the Oostvoorne–Europoort population was assumed to be compensated by both other Oostvoorne populations.
Transition scenarios
As a starting point for all the simulations we used the present situation based on the actual data collected by RIKZ and INBO (Table 1). We changed the present habitat configuration to an alternative scenario 50 years after the start of the simulation.
To investigate the importance of overlap between loss of breeding habitat and newly created habitat, we formulated seven transition scenarios for each main scenario, representing different transitions of breeding habitat after 50 years:
-
(a)
At year 50, new habitats become suitable whilst the old habitats remain suitable for another two decades, so a 20-year overlap is created during which old and new habitats coexist (20-year overlap scenario)
-
(b)
An 8-year overlap scenario.
-
(c)
A 3-year overlap scenario.
-
(d)
At year 50, the old habitats become unsuitable and at the same time alternative breeding habitats become suitable to replace them (consecutive scenario).
-
(e)
At year 50, the old habitats become unsuitable and 3 years later, newly created habitats become suitable, thus leaving a gap of 3 years (3-year gap scenario).
-
(f)
An 8-year gap scenario.
-
(g)
A 20-year gap scenario.
We ran each scenario 50 times to obtain insight into variations in model outcomes.
Model sensitivity
To examine the robustness of our approach we investigated model response with respect to parameter change. We did this for two main population dynamical parameters: adult mortality (1−S a ) and recruitment (R e ), and for the four main dispersal parameters: the distance decay exponent (α), the dispersal fraction at carrying capacity (F cc ), the density dependence exponent (e) for the dispersal, and the critical density value to start a population (C p ) (Table 3, S1 in ESM). We tested sensitivity in the consecutive case of scenario (2d) and evaluated the sensitivity on the mean final population level and on the return time to the 95% equilibrium level after the habitat patch replacement. We evaluated the % response divided by the % parameter change according to Schippers and Kropff (2001).
Results
Common Tern data analysis
According to the RIKZ and INBO data, Common Terns were observed breeding at 162 locations in the Dutch–Belgian delta region during 1991–2005. Most of those locations show large fluctuations in numbers of breeding pairs over the years (Fig. 2). Overall, despite the large fluctuations in local populations, the total delta metapopulation was quite stable during the study period, with the total number of Common Terns varying between 5,000 and 10,000 breeding pairs between 1991 and 2005 (Fig. 3). It is worth mentioning that a small set of just 10 locations (see Table 1) accommodated more than 72% of the total Dutch–Belgian delta population, with 544 breeding pairs per location on average, whereas 100 other locations accommodated fewer than 10 breeding pairs. The metapopulation can therefore be aptly described as a network of a few large and stable populations surrounded by many small populations that are frequently empty. Numbers of breeding pairs in port areas showed an increasing trend, starting at 25% in the early 1990s and reaching 40% in 2005 (Fig. 3).
Measured Common Tern breeding pair numbers per location. Note the large fluctuations in numbers through the years. For locations see Table 1
Simulation results of scenarios
Scenario 1: no sites in ports
Simulations for this scenario show trends over the first 50 years similar to the actual situation observed in the 1991–2005 period (Figs. 3 and 4a). At the level of individual populations the model results also show fluctuations similar to those observed in existing colonies. When the port colonies disappear the average population level drops from 7,600 pairs to 4,500 pairs, a decrease of 41%. Though the metapopulation fluctuates between some 4,500 and 6,500 pairs, it never goes extinct, although the population size sometimes reaches a level of as few as 2,500 pairs. The drop in the average size—compared to the current situation—is as large as the maximum number of pairs in all of the port habitats together, which is relatively high. If we had left out all the non-natural breeding sites (including, e.g., the habitats in the Port of Terneuzen) the situation would have been even worse, with extinction possible.
Simulation results for the different scenarios: a current situation followed by removal of all port habitats, b current situation followed by port habitat removal with compensation in the same port, c current situation followed by removal of port habitat compensated for near the same port while increasing the carrying capacity, d current situation followed by removal of port habitat compensated for in central locations in the metapopulation while increasing the carrying capacity. Simulations were performed under the following conditions: 20-year overlap, 8-year overlap, 3-year overlap, consecutive, 3-year gap, 8-year gap, and 20-year gap
Scenario 2: compensation in ports
In scenario 2, lost habitats are compensated for within the ports (Fig. 4b). Here, the simulations with 20 years overlap of old and newly created breeding habitats (scenario 2a) show the metapopulation increasing during the first 20 years after compensation and thereafter decreasing to the equilibrium condition of 7,600 breeding pairs. In the consecutive scenario (2d), the metapopulation decreases to 6,300 breeding pairs in the first 20 years after habitat change. It takes the metapopulation 40 years to recover to a level of 7,600 pairs. In the gap scenario (2g), where the old habitats are replaced 20 years after they are lost, the decline is larger, yielding a metapopulation of 5,000 breeding pairs. Surprisingly, this scenario very slowly increases to a level of 5,500 breeding pairs in 200 years after the habitat replacement.
Scenario 3: compensation near ports
In scenario 3, we test the impact on the Common Tern metapopulation of suitable replacement sites being provided outside but near the port areas (Fig. 4c). In the overlap scenario (3a), the metapopulation vastly increases to 8,900 breeding pairs, but when the old habitats are removed the average metapopulation declines a little bit. After a few years the population starts to grow, reaching 9,100 breeding pairs 40 years after the habitat transition. In the consecutive scenario (3d), the metapopulation plummets to a level of 6,600 breeding pairs. After 10 years, however, the population starts to grow, reaching 9,000 breeding pairs 70 years after the replacement was executed. In scenario (3g), having a gap of 20 years between loss of old habitat and its replacement with new causes the population to drop to 5,000 pairs over 30 years. Subsequently, the population starts a slow increase, reaching the new equilibrium level of 9,000 in 200 years.
Scenario 4: compensation central
When replacement sites are located near the heart of the Common Tern metapopulation, the simulations roughly match those of the previous scenario (compare Fig. 4c and d). However, the few differences are worth noting. The recovery to equilibrium after habitat replacement is much slower in scenario 4, but the final equilibrium level is 500 breeding pairs higher compared to scenario 3.
Sensitivity analysis
Adult mortality and recruitment are the most sensitive parameters with respect to the population level (Table 3). This was also true for the return time because higher population levels will cause more dispersal and stimulate colonization of new habitat. The model was not very sensitive for a change in the dispersal related parameters (Table 3). This can be understood from the fact that increased dispersal also reduces levels of well-reproducing populations. Here, increased dispersal has a draw back on population dynamics.
Discussion
The most striking simulation result is from the compensation in ports scenario (2g), in which, when a 20-year gap separates the disappearance of the old habitats and the creation of new habitats, the metapopulation completely fails to recover. This is due to colonization limitations. Birds are unable to start up a growing population after they leave the port area because the newly created habitat patches are small in dimension and are located near the edge of the metapopulation. The Common Tern favors the company of congeners (Dittmann et al. 2005). Their populations have relatively low per capita growth rates at low densities due to increased predation of chicks (Krebs and Davies 1978; Becker 1984; Cavanagh and Griffin 1993; Whittam and Leonard 2000; Becker and Ludwigs 2004; Serrano et al. 2005), and smaller colonies means less effective foraging (Buckley 1997). These factors make it difficult to recolonize the new port habitat at a metapopulation level of just 5,500 breeding pairs retracted to the centre of the metapopulation. The low reproduction at low densities cause individual population to have alternative stable states (see, e.g., Scheffer et al. 2001; Schippers et al. 2006) one without any animals and another with a considerable amount of animals causing alternative attractors in the model, at 5,500 and 7,600 breeding pairs, respectively (Fig. 4b).
These results raise the question of how these port colonies developed in the first place, because in 1993 and 1994 the number of breeding pairs was actually less than 5,500 pairs. This may be explained by the different spatial distribution of the birds in 1993 and 1994 from that in the model after the removal of the breeding habitats in the ports. In the model, no bird could breed for 20 years in or near the port areas, whereas in 1993 and 1994, 25–30% of the breeding pairs were still breeding in the ports, and no recolonization from elsewhere was necessary because the birds were still present. These alternative attractors are thus dependent not only on the number of animals in the metapopulation but also on the spatial distribution of the breeding pairs. Other scenarios describing compensation in ports, the 3-year gap (2c), the consecutive (2d), and the overlap scenarios (2e–g), however, do converge to the other stable equilibrium of 7,600 breeding pairs, indicating that an average population size of 6,000 is enough for expansion to 7,600 pairs. In these scenarios, the habitat compensation was successful.
In the scenarios in which we seek compensation outside of the port areas (scenarios 3 and 4) and in which we enlarge the total carrying capacity, the picture roughly matches that of the compensation within the port scenario (2). The metapopulation levels, however, do not gradually increase over time to their stable equilibrium. Here, we must realize that we are dealing with average values of 100 simulations (Fig. 4). In these scenarios, however, there are also two equilibria: at 6,000 and at about 9,000 breeding pairs. The reason for the steady increase is that the population returns to the 6,000 equilibrium in the case where the population first drops, and subsequently individual simulations, as in scenario 2 (Fig. 5), swap from the 6,000 to the 9,000 attraction basin and do not return. These switches over time, in turn, are responsible for the gradual increase of the average number and are induced by an accidental sequence of good years (no flooding of breeding habitat). This pushes the metapopulations out of the attraction zone of the 6,000 equilibrium into the attraction zone of 9,000 pairs.
There are marked differences in the results of the different transition scenarios. Having a 20-year overlap leads to a temporary increase that is not lost in the scenarios with expanded carrying capacity (scenarios 3 and 4). Here, a larger percentage of the simulations already swap to the 9,000 equilibrium and do not return as a result of the abandonment of the old breeding habitats. In the consecutive and gap scenarios, large drops occur at the metapopulation level followed by no or very slow regeneration. We conclude, therefore, that having overlap is key, since temporary drops in population numbers might result in very slow recovery. In our simulations, the central compensation scenario (4) performed best because the new populations at the heart of the metapopulation were easily found by dispersers, which improved initial resilience and final carrying capacity. These results suggest that the location of newly created habitat matters with respect to the resilience of the metapopulation.
That the creation of alternative breeding sites can be successful has been demonstrated by the flourishing of ‘Tern Peninsula’ (Sternenschiereiland) in the Port of Zeebrugge (Belgium). This newly created landmass covering about 10 ha shows that artificial breeding habitats created near existing populations can prosper. In 2004, 1,832 Common Terns, 138 Little Terns (Sterna albifrons) and 4,067 Sandwich Terns (Sterna sandvicensis) were observed breeding on this peninsula (Courtens et al. 2008). Part of this population presumably originated from an existing location in the Port of Zeebrugge. This experiment underlines the role that human-made habitats could play in the protection of endangered costal birds, especially in areas like ports, where the Common Tern’s nesting behavior might conflict with, for example, port development. However, we know from other cases that offering alternative breeding sites for the Common Tern is not always successful (Meininger and Graveland 2002).
During the period 1991–2005, the proportion of the delta metapopulation of Common Terns nesting in port areas increased from 25 to 40%. This is largely explained by the fact that natural sites are rare in the delta at present, and birds depend mainly on semi-natural sites like embankments created for coastal defence and the new port areas. Most of the remaining natural sites, such as beaches, are too disturbed by recreational activities to accommodate bird colonies. Port areas have therefore become increasingly important as breeding sites for the Common Tern. Our results suggest that, if the breeding sites located within port areas were lost, the size of the delta metapopulation of Common Terns would seriously decline, though without leading to overall extinction. If, to replace the lost habitat, additional habitat patches were created within the port area as alternative breeding sites, loss of the current sites would lead to only a small decrease of the delta metapopulation. However, if this additional habitat were developed outside the port area, where there is more space for nesting birds, the end result might be positive with respect to the size of the metapopulation. Clearly, food availability at any newly created sites should be abundant enough to sustain the breeding birds, and there should be a considerable overlap of old and new breeding habitat.
In conclusion, our model describing metapopulation dynamics of the Common Tern gives realistic results in the scenario that we can test. Our results suggest that creation of new safe breeding habitat is a promising way to compensate for lost habitat. However, we should keep in mind that food security, during the breeding period will largely determine the success of the new habitat sites. Furthermore, our results show that a considerable overlap in time between the loss of old and availability of new breeding habitat is necessary to ensure that newly created breeding sites do start up. Additionally, unlike the view taken in legislation like the EU Bird Directive, we believe that conservation of seabirds should be done on a metapopulation scale rather than a local scale, because bird protection on a local scale may obstruct good protection measures at the regional level, like the creation of suitable alternative breeding sites. On the other hand, our results agree with the EU Bird Directive in that old habitats should first be compensated for before being destroyed.
References
Akçakaya HR, Atwood JL, Breininger D, Collins CT, Duncan B (2003) Metapopulation dynamics of the California least tern. J Wildl Manage 67:829–842. doi:10.2307/3802690
Alonso JC, Martin CA, Alonso JA, Palacin C, Magana M, Lane SJ (2004) Distribution dynamics of a great bustard metapopulation throughout a decade: influence of conspecific attraction and recruitment. Biodivers Conserv 13:1659–1674. doi:10.1023/B:BIOC.0000029329.44373.47
Becker PH (1984) How does the Common Tern (Strena hirudo) organise its defence against Herring Gull (Larus argentatus)? Z Tierpsychol 66:265–288 In German
Becker PH, Bredley JS (2007) The role of intrinsic factors for the recruitment process in long-lived birds. J Ornithol 148:S377–S384. doi:10.1007/s10336-007-0157-x
Becker PH, Ludwigs JD (2004) Sterna hirundo Common Tern. BWP Update 6(1&2):91–137
Becker PH, Frank D, Sudmann SR (1993) Temporal and spatial pattern of common tern (Sterna hirundo) foraging in the Wadden Sea. Oecologia 93:389–393. doi:10.1007/BF00317883
Buckley NJ (1997) Spatial-concentration effects and the importance of local enhancement in the evolution of colonial breeding in seabirds. Am Nat 149:1091–1112. doi:10.1086/286040
Cam E, Oro D, Pradel R, Jimenez J (2004) Assessment of hypotheses about dispersal in a long-lived seabird using multistate capture-recapture models. J Anim Ecol 73:723–736. doi:10.1111/j.0021-8790.2004.00848.x
Cavanagh PM, Griffin CR (1993) Responses of nesting common terns and laughing gulls to flyovers by large gulls. Wilson Bull 105:333–338
Courtens W, Stienen EWM, Van de Walle M (2007) The breeding season 2007 in Zeebrugge: a first impression. Vogelnieuws: Ornithologische Nieuwsbrief van het Instituut voor Natuur-en Bosonderzoek 8:1–16 (in Dutch)
Courtens W, Stienen EWM, Van de Walle M (2008) Terns at Zeebrugge: breeding on an artificial peninsula. Mens & Vogel 46:30–37 (in Dutch)
Dittmann T, Zinsmeister D, Becker PH (2005) Dispersal decisions: Common terns, Sterna hirundo, choose between colonies during prospecting. Anim Behav 70:13–20. doi:10.1016/j.anbehav.2004.09.015
Erwin RM, Hatfield JS, Wilmers TJ (1995) The value and vulnerability of small estuarine islands for conserving metapopulations of breeding waterbirds. Biol Conserv 71:187–191. doi:10.1016/0006-3207(94)00045-R
Erwin RM, Nichols JD, Eyler TB, Stotts DB, Truitt BR (1998) Modeling colony-site dynamics: a case study of gull-billed terns (Sterna nilotica) in coastal Virginia. Auk 115:970–978
Hanski I, Gilpin M (1991) Metapopulation dynamics: brief-history and conceptual domain. Biol J Linn Soc Lond 42:3–16. doi:10.1111/j.1095-8312.1991.tb00548.x
Jones HP, Tershy BR, Zavaleta ES, Croll DA, Keitt BS, Finkelstein ME, Howald GR (2008) Severity of the effects of invasive rats on seabirds: a global review. Conserv Biol 22:16–26. doi:10.1111/j.1523-1739.2007.00859.x
Krebs JB, Davies NB (1978) Behavioural ecology: an evolutionary approach. Blackwell, Oxford
Levins R (1970) Extinction. In: Gerstenhauber M (ed) Some mathematical questions in biology, vol 2. American Mathematical Society, Providence, pp 77–107
Martinez-Abrain A, Oro D, Forero MG, Conesa D (2003) Modeling temporal and spatial colony-site dynamics in a long-lived seabird. Popul Ecol 45:133–139. doi:10.1007/s10144-003-0150-z
Meininger PL, Graveland J (2002) Directive ecological recovery measures for coastal breeding birds: balancing between natural processes and intervention. Report nr. 2001.046. RIKZ, Middelburg (in Dutch)
Meininger PL, Arts FA, Van Swelm ND (2000) Coastal breeding birds in the northern Delta area: developments, bottlenecks and possibilities. Report nr. 2000.052. RIKZ, Middelburg (in Dutch)
Morales MB, Bretagnolle V, Arroyo B (2005) Viability of the endangered little bustard (Tetrax tetrax) population of western France. Biodivers Conserv 14:3135–3150. doi:10.1007/s10531-004-0382-z
Opdam P (1991) Metapopulation theory and habitat fragmentation: a review of holarctic breeding bird studies. Landsc Ecol 5:93–106. doi:10.1007/BF00124663
Oro D (2003) Managing seabird metapopulations in the Mediterranean: constraints and challenges. Sci Mar 67:13–22. doi:10.3989/scimar.2003.67s213
Oro D, Cam E, Pradel R, Martinez-Abrain A (2004) Influence of food availability on demography and local population dynamics in a long-lived seabird. Proc R Soc Lond B 271:387–396. doi:10.1098/rspb.2003.2609
Pulliam HR (1988) Sources, sinks, and population regulation. Am Nat 132:652–661. doi:10.1086/284880
Sachot S, Perrin N, Neet C (2006) Viability and management of an endangered Capercaillie (Tetrao urogallus) metapopulation in the Jura Mountains, Western Switzerland. Biodivers Conserv 15:2017–2032. doi:10.1007/s10531-005-0771-y
Scheffer M, Carpenter S, Foley JA, Folke C, Walker B (2001) Catastrophic shifts in ecosystems. Nature 413:591–596. doi:10.1038/35098000
Schippers P, Kropff MJ (2001) Competition for light and nitrogen among grassland species: a simulation analysis. Funct Ecol 15:155–164. doi:10.1046/j.1365-2435.2001.00509.x
Schippers P, Verboom J, Knaapen JP, Van Apeldoorn RC (1996) Dispersal and habitat connectivity in complex heterogeneous landscapes: an analysis with a GIS-based random walk model. Ecography 19:97–106. doi:10.1111/j.1600-0587.1996.tb00160.x
Schippers P, Van de Weerd H, De Klein J, De Jong B, Scheffer M (2006) Impacts of agricultural phosphorus use in catchments on shallow lake water quality: about buffers, time delays and equilibria. Sci Total Environ 369:280–294. doi:10.1016/j.scitotenv.2006.04.028
Schroder SE, Schobben JHM, Meininger PL (1996) A population model for the common Tern (Sterna hirundo). RIKZ, Middelburg
Serrano D, Tella JL (2003) Dispersal within a spatially structured population of lesser kestrels: the role of spatial isolation and conspecific attraction. J Anim Ecol 72:400–410. doi:10.1046/j.1365-2656.2003.00707.x
Serrano D, Oro D, Ursua E, Tella JL (2005) Colony size selection determines adult survival and dispersal preferences: allee effects in a colonial bird. Am Nat 166:E22–E31. doi:10.1086/431255
Spendelow JA, Nichols JD, Nisbet ICT, Hays H, Cormons GD, Burger J, Safina C, Hines JE, Gochfeld M (1995) Estimating annual survival and movement rates of adults within a metapopulation of Roseate Terns. Ecology 76:2415–2428. doi:10.2307/2265817
Stienen EWM, Brenninkmeijer A (1992) Ecological profile of the Common Tern (Sterna hirundo). RIN-report 92(18), Instituut voor Bos- en Natuuronderzoek, DLO-Institute for Forest and Nature conservation, Arnhem
Stienen EWM, Brenninkmeijer A (2002) Variation in growth in Sandwich Tern chicks Sterna sandvicensis and the consequences for pre- and post-fledging mortality. Ibis 144:567–576. doi:10.1046/j.1474-919X.2002.00086.x
Stienen EWM, Brenninkmeijer A (2006) Effect of brood size and hatching sequence on prefledging mortality of Sandwich terns: why lay two eggs? J Ornithol 147:520–530. doi:10.1007/s10336-006-0075-3
Stienen EWM, Van Beers PWM, Brenninkmeijer A, Habraken J, Raaijmakers M, Van Tienen PGM (2000) Reflections of a specialist: patterns in food provisioning and foraging conditions in sandwich terns (Sterna sandvicensis). Ardea 88:33–49
Stienen EWM, Courtens W, Van de Walle M, Van Waeyenberge J, Kuijken E (2005) Harbouring nature: port development and dynamic birds provide clues for conservation. In: International conference on nature restoration practices in European Coastal Koksijde, 19–23 September 2005
Strucker RCW, Hoekstein MSJ, Meininger PL (2005) Coastal breeding birds in the Delta area in 2004, with a summary of 2003. Report nr. 2005.016, RIKZ, Middelburg
Van Apeldoorn RC, Knaapen JP, Schippers P, Verboom J, Van Engen H, Meeuwsen H (1998) Applying ecological knowledge in landscape planning: a simulation model as a tool to evaluate scenarios for the badger in the Netherlands. Landsc Urban Plan 41:57–69. doi:10.1016/S0169-2046(97)00058-3
Van der Hoorn B, Meininger PL, Wattel J (1997) A contribution to the population dynamics of the Common Tern. Report nr. OS-97.815X, RIKZ, Middelburg
Verboom J, Schotman A, Opdam P, Metz JAJ (1991) European nuthatch metapopulations in a fragmented agricultural landscape. Oikos 61:149–156. doi:10.2307/3545332
Verboom J, Foppen R, Chardon P, Opdam P, Luttikhuizen P (2001) Introducing the key patch approach for habitat networks with persistent populations: an example for marshland birds. Biol Conserv 100:89–101. doi:10.1016/S0006-3207(00)00210-X
Vermaat JE, Vigneau N, Omtzigt N (2008) Viability of meta-populations of wetland birds in a fragmented landscape: testing the key-patch approach. Biodivers Conserv 17:2263–2273. doi:10.1007/s10531-008-9401-9
Vos CC, Verboom J, Opdam PFM, Ter Braak CJF (2001) Toward ecologically scaled landscape indices. Am Nat 157:24–41. doi:10.1086/317004
Whittam RM, Leonard ML (2000) Characteristics of predators and offspring influence nest defence by Arctic and Common Terns. Condor 102:301–306. doi:10.1650/0010-5422(2000)102[0301:COPAOI]2.0.CO;2
Wiens JA (1989) Ecology of bird communities. Volume 1: foundations and patterns. Cambridge University Press, Cambridge
Ysebaert T, Meininger PL, Meire P, Devos K, Berrevoets CM, Strucker RCW, Kuijken E (2000) Waterbird communities along the estuarine salinity gradient of the Schelde estuary, NW-Europe. Biodivers Conserv 9:1275–1296. doi:10.1023/A:1008976306651
Acknowledgments
We thank Jan Willem van Veen and Arjan Griffioen for their help in digesting the data and drawing Fig. 1. The former Dutch National Institute for Sea and Marine Management (RIKZ; nowadays ‘Waterdienst’) and the Belgian Research Institute for Nature and Forest (INBO) provided valuable data from their intensive monitoring programmes. We thank these Institutes for their important contribution. Concerning the Dutch bird breeding data, these originated from the Saltwater Biological Monitoring Programme of the RIKZ, as part of the ‘State of the Nation’ Waterworks Monitoring Programme (Monitoring-Programma Waterstaatkundige Toestand van het Land (MWTL)) and finally we thank Michelle Luijben for improving our English.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License ( https://creativecommons.org/licenses/by-nc/2.0 ), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Schippers, P., Snep, R.P.H., Schotman, A.G.M. et al. Seabird metapopulations: searching for alternative breeding habitats. Popul Ecol 51, 459–470 (2009). https://doi.org/10.1007/s10144-009-0159-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10144-009-0159-z