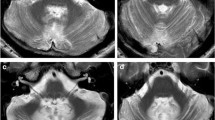
Abstract
The dentato-rubro-olivary pathway, also known as the Guillain-Mollaret triangle (GMT) or myoclonic triangle, consists of the dentate nucleus, the red nucleus, and the inferior olivary nucleus (ION). GMT is important for motor coordination and control, and abnormalities in this network can lead to various neurological disorders. The present study followed a systematic approach in conducting a review on GMT studies. The inclusion criteria were limited to human subjects with primary objectives of characterizing and evaluating GMT syndromes, and the methodology used was not a determining factor for eligibility. The search strategy used MeSH terms and keywords relevant to the study’s objective in various databases until August 2022. A total of 76 studies were included in the review after assessing 527 articles for eligibility based on the final inclusion criteria. Most of the studies evaluated the GMT in human subjects, with the majority utilizing magnetic resonance imaging (MRI), diffusion tensor imaging (DTI), or combination of them. The review found that Hypertrophic olivary degeneration (HOD), a common consequence of GMT damage, has diverse underlying causes, including stroke, brainstem cavernous malformations, and structural impairments. Palatal tremor, ocular myoclonus, ataxia, nystagmus, and vertigo were frequently reported symptoms associated with HOD. This systematic review provides comprehensive insights into the association between GMT and various neurological syndromes, shedding light on the diagnostic, etiological, and prognostic aspects of GMT dysfunction. Understanding the role of the GMT and its implications in movement disorders could pave the way for improved treatment options and better management of neurological conditions related to this critical brainstem pathway.


Similar content being viewed by others
Data availability
Data and materials are available for review upon request.
References
Guillain G (1931) Deux cas de myoclonies synchrones et rythmées vélo-pliaryngo-oculo-diaphragmatiques. Rev Neurol 2:545–546
Turgut AÇ, Tubbs RS, Turgut M (2019) Georges Charles Guillain (1876–1961) and Pierre Mollaret (1898–1987) and their legacy to neuroanatomy: the forgotten triangle of Guillain-Mollaret. Childs Nerv Syst 37:349–350. https://doi.org/10.1007/s00381-018-04033-8
Lavezzi AM, Corna M, Matturri L, Santoro F (2009) Neuropathology of the Guillain-Mollaret Triangle (Dentato-Rubro-Olivary Network) in sudden unexplained perinatal death and SIDS. Open Neurol J 3:48–53. https://doi.org/10.2174/1874205x00903010048
Dincer A, Ozyurt O, Kaya D, Kosak E, Ozturk C, Erzen C, Pamir MN (2011) Diffusion tensor imaging of Guillain-Mollaret triangle in patients with hypertrophic olivary degeneration. J Neuroimaging 21:145–151. https://doi.org/10.1111/j.1552-6569.2009.00461.x
Elnekiedy A, Naguib N, Hamed W, Mekky J, Mamdouh Hassan HH (2016) MRI and neurological presentation of hypertrophic olivary degeneration. Egypt J Radiol Nucl Med 47:1019–1029. https://doi.org/10.1016/j.ejrnm.2016.04.019
Goyal M, Versnick E, Tuite P, Cyr JS, Kucharczyk W, Montanera W, Willinsky R, Mikulis D (2000) Hypertrophic olivary degeneration: metaanalysis of the temporal evolution of MR findings. AJNR Am J Neuroradiol 21:1073–1077
Avula S, Spiteri M, Kumar R, Lewis E, Harave S, Windridge D, Ong C, Pizer B (2016) Post-operative pediatric cerebellar mutism syndrome and its association with hypertrophic olivary degeneration. Quant Imaging Med Surg 6:535–544. https://doi.org/10.21037/qims.2016.10.11
Smets G, Lambert J, Tijssen M, Mai C, Decramer T, Vandenberghe W, Van Loon J, Demaerel P (2017) The dentato-rubro-olivary pathway revisited: new MR imaging observations regarding hypertrophic olivary degeneration. Clin Anat 30:543–549. https://doi.org/10.1002/ca.22866
Haddaway NR, Page MJ, Pritchard CC, McGuinness LA (2022) PRISMA2020: an R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev 18:e1230. https://doi.org/10.1002/cl2.1230
Ogut E, Armagan K (2022) Evaluation of the potential impact of medical ozone therapy on Covid-19: a review study. Ozone Sci Eng 45(3):213–231. https://doi.org/10.1080/01919512.2022.2065242
Ogut E, Karakas O, Aydin DD (2022) Oppenheimer's accessory ossicle and clinical significance: a narrative review. J Orthop Rep 1:100069. https://doi.org/10.1016/j.jorep.2022.100069
Ogut E (2022) The Stieda process of the talus: the anatomical knowledge and clinical significance of an overlooked protrusion. Bull Natl Res Cent 46:280. https://doi.org/10.1186/s42269-022-00968-w
Ogut E (2022) Is the third trochanter of the femur a developmental anomaly, a functional marker, or an evolutionary adaptation? Can Soc Forensic Sci J:1–20. https://doi.org/10.1080/00085030.2022.2104563
Ogut E, Armagan K, Gül Z (2022) The role of syringic acid as a neuroprotective agent for neurodegenerative disorders and future expectations. Metab Brain Dis 37:859–880. https://doi.org/10.1007/s11011-022-00960-3
Karsan N, Reitboeck PG, Lambert C, Omer S (2016) Palatal tremor: 12 cases-the experience of a tertiary neuroscience centre. J Neurol Neurosurg Psychiatry 87:e1.5. https://doi.org/10.1136/jnnp-2016-315106.102
Deusch G, Toro C, Valls-Solé J, Zeffiro T, Zee DS, Hallett M (1994) Symptomatic and essential palatal tremor. Brain 117:775–788. https://doi.org/10.1093/brain/117.4.775
Lee S, Moon HI, Shin J-H (2021) Post-stroke palatal tremor as a clinical predictor of dysphagia and its neuroanatomical correlates in patients with midbrain and pontine lesions. J Neural Transm 128:1863–1872. https://doi.org/10.1007/s00702-021-02417-w
Jang L, Borruat F-X (2014) Oculopalatal tremor: variations on a theme by Guillain and Mollaret. Eur Neurol 72:144–149. https://doi.org/10.1159/000360531
Kim JS, Moon SY, Choi KD, Kim JH, Sharpe JA (2007) Patterns of ocular oscillation in oculopalatal tremor: imaging correlations. Neurology 68:1128–1135. https://doi.org/10.1212/01.wnl.0000258665.37827.f6
Liao K, Hong S, Zee DS, Optican LM, Leigh RJ (2008) Impulsive head rotation resets oculopalatal tremor: examination of a model. Prog Brain Res 171:227–234. https://doi.org/10.1016/s0079-6123(08)00632-8
Shaikh AG, Ghasia FF, DeLong MR, Jinnah HA, Freeman A, Factor SA (2015) Ocular Palatal tremor plus dystonia: new syndromic association. Mov Disord Clin Pract 2:267–270. https://doi.org/10.1002/mdc3.12193
Jabbari B, Rosenberg M, Scherokman B, Gunderson CH, McBurney JW, McClintock W (1987) Effectiveness of trihexyphenidyl against pendular nystagmus and palatal myoclonus: evidence of cholinergic dysfunction. Mov Disord 2:93–98. https://doi.org/10.1002/mds.870020202
Mastaglia FL, Grainger MR, Kakulas BA (1974) Palatal myoclonus and associated movements. Proc Aust Assoc Neurol 11:183–187
Seidman MD, Arenberg JG, Shirwany NA (1999) Palatal myoclonus as a cause of objective tinnitus: a report of six cases and a review of the literature. Ear Nose Throat J 78:292–297
Krause E, Heinen F, Gürkov R (2010) Difference in outcome of botulinum toxin treatment of essential palatal tremor in children and adults. Am J Otolaryngol 31:91–95. https://doi.org/10.1016/j.amjoto.2008.11.007
Samuel M, Torun N, Tuite PJ, Sharpe JA, Lang AE (2004) Progressive ataxia and palatal tremor (PAPT): clinical and MRI assessment with review of palatal tremors. Brain 127:1252–1268. https://doi.org/10.1093/brain/awh137
Carr CM, Hunt CH, Kaufmann TJ, Kotsenas AL, Krecke KN, Wood CP (2015) Frequency of bilateral hypertrophic olivary degeneration in a large retrospective cohort. J Neuroimaging 25:289–295. https://doi.org/10.1111/jon.12118
Derner M, Drugová B, Druga R (2009) Inferior olive hypertrophy. Cesk Radiol 63:232–237
Hanihara T, Amano N, Takahashi T, Itoh Y, Yagishita S (1998) Hypertrophy of the inferior olivary nucleus in patients with progressive supranuclear palsy. Eur Neurol 39:97–102. https://doi.org/10.1159/000007915
Blanco Ulla M, López Carballeira A, Pumar Cebreiro JM (2015) Imagen por resonancia magnética en la degeneración olivar hipertrófica. Radiología 57:505–511. https://doi.org/10.1016/j.rx.2014.12.008
Conceicao C, Palma T, Cravo I, Graca J, Ribeiro C (2001) Hypertrophic olivary degeneration. Semiology with magnetic resonance. Acta Med Port 14:107–111
Foerch C, Schaller MA, Lapa S, Filipski K, Steinmetz H, Kang J-S, Zöllner JP, Wagner M (2018) Hypertrophe Degeneration der Olive. Nervenarzt 90:609–615. https://doi.org/10.1007/s00115-018-0646-6
Gu CN, Carr CM, Kaufmann TJ, Kotsenas AL, Hunt CH, Wood CP (2015) MRI findings in nonlesional hypertrophic olivary degeneration. J Neuroimaging 25:813–817. https://doi.org/10.1111/jon.12267
Kim SJ, Lee JH, Suh DC (1994) Cerebellar MR changes in patients with olivary hypertrophic degeneration. AJNR Am J Neuroradiol 15:1715–1719
Kitajima M, Korogi Y, Shimomura O, Sakamoto Y, Hirai T, Miyayama H, Takahashi M (1994) Hypertrophic olivary degeneration: MR imaging and pathologic findings. Radiology 192:539–543. https://doi.org/10.1148/radiology.192.2.8029428
Konno T, Broderick DF, Tacik P, Caviness JN, Wszolek ZK (2016) Hypertrophic olivary degeneration: a clinico-radiologic study. Parkinsonism Relat Disord 28:36–40. https://doi.org/10.1016/j.parkreldis.2016.04.008
Sabat S, Mannering N, Agarwal A (2016) Hypertrophic olivary degeneration: case series and review of literature. J Neurol Sci 370:180–186. https://doi.org/10.1016/j.jns.2016.09.055
Suzuki M, Takashima T, Ueda F, Fujinaga Y, Horichi Y, Yamashita J (1999) Olivary degeneration after intracranial haemorrhage or trauma: follow-up MRI. Neuroradiology 41:9–12. https://doi.org/10.1007/s002340050695
Behzadi F, Fiester PJ, Rao D (2021) Bilateral hypertrophic olivary degeneration following brainstem insult: a retrospective review and examination of causative pathology. Neurosci Insights 16:26331055211007445. https://doi.org/10.1177/26331055211007445
Wolin MJ, Trent RG, Lavin PJM, Cornblath WT (1996) Oculopalatal myoclonus after the one-and-a-half syndrome with facial nerve palsy. Ophthalmology 103:177–180. https://doi.org/10.1016/s0161-6420(96)30743-4
Zhang H, Fu Y, Li X, Dong R-F, Yin J-N, He P-P, Gao Y, Wang Y-L (2020) A meta-analysis of case studies and clinical characteristics of hypertrophic olivary degeneration secondary to brainstem infarction. J Integr Neurosci 19(3):507–511. https://doi.org/10.31083/j.jin.2020.03.1238
Arora A, Kapoor A, Gupta R, Puri SK (2010) Guillain-Mollaret triangle &hypertrophic olivary degeneration, vol 1. EuroRad, p 8764. https://doi.org/10.1594/EURORAD/CASE.8764
Auffray-Calvier E, Desal HA, Naudou-Giron E, Severin-Fontana S, Cavenaile-Dolez H, Stefan A, Doury E, de Kersaint-Gilly A (2005) Hypertrophic olivary degeneration. MR imaging findings and temporal evolution. J Neuroradiol 32:67–72. https://doi.org/10.1016/s0150-9861(05)83026-3
Biancheri R, Rossi A, Veneselli E, De Grandis E, Striano P, Di Rocco M, Bruno C, Morana G, Mirabelli-Badenier M (2015) Inferior olivary nucleus involvement in pediatric neurodegenerative disorders: does it play a role in neuroimaging pattern-recognition approach? Neuropediatrics 46:104–109. https://doi.org/10.1055/s-0035-1544185
Bindu PS, Taly AB, Sonam K, Govindaraju C, Arvinda HR, Gayathri N, Bharath MM, Ranjith D, Nagappa M, Sinha S, Khan NA, Thangaraj K (2014) Bilateral hypertrophic olivary nucleus degeneration on magnetic resonance imaging in children with Leigh and Leigh-like syndrome. Br J Radiol 87:20130478. https://doi.org/10.1259/bjr.20130478
Boesten AJP, Voogd J (1985) Hypertrophy of neurons in the inferior olive after cerebellar ablations in the cat. Neurosci Lett 61:49–54. https://doi.org/10.1016/0304-3940(85)90399-4
Hirano M, Hatzoglou V, Karimi S, Young RJ (2015) Hypertrophic olivary degeneration resulting from posterior fossa masses and their treatments. Clin Imaging 39:787–790. https://doi.org/10.1016/j.clinimag.2015.05.015
Hornyak M, Osborn AG, Couldwell WT (2008) Hypertrophic olivary degeneration after surgical removal of cavernous malformations of the brain stem: report of four cases and review of the literature. Acta Neurochir 150:149–156. https://doi.org/10.1007/s00701-007-1470-0
Jellinger K (1973) Hypertrophy of the inferior olives. Z Neurol 205:153–174. https://doi.org/10.1007/bf00316018
Katsuse O, Dickson D (2004) Inferior olivary hypertrophy is uncommon in progressive supranuclear palsy. Acta Neuropathol 108:143–146. https://doi.org/10.1007/s00401-004-0878-3
Martins WA, Marrone LC, Soder RB, Costa JC (2016) Hypertrophic olivary degeneration: unveiling the triangle of Guillain-Mollaret. Arq Neuropsiquiatr 74:426–427. https://doi.org/10.1590/0004-282X20160049
Onen MR, Moore K, Cikla U, Ucer M, Schmidt B, Field AS, Baskaya MK (2018) Hypertrophic olivary degeneration: neurosurgical perspective and literature review. World Neurosurg 112:e763–e771. https://doi.org/10.1016/j.wneu.2018.01.150
Otto J, Guenther P, Hoffmann K-T (2013) Bilateral hypertrophic olivary degeneration in Wilson disease. Korean J Radiol 14:316–320
Ruigrok TJ, de Zeeuw CI, Voogd J (1990) Hypertrophy of inferior olivary neurons: a degenerative, regenerative or plasticity phenomenon. Eur J Morphol 28:224–239
Sánchez Hernández J, Paniagua Escudero JC, Carreño Morán P, Asensio Calle JF (2013) Hypertrophic olivary degeneration secondary to a Guillain–Mollaret triangle lesion. Neurología 28:59–61. https://doi.org/10.1016/j.nrleng.2011.04.008
Sanverdi SE, Oguz KK, Haliloglu G (2012) Hypertrophic olivary degeneration in children: four new cases and a review of the literature with an emphasis on the MRI findings. Br J Radiol 85:511–516. https://doi.org/10.1259/bjr/60727602
Schaller-Paule MA, Steidl E, Shrestha M, Deichmann R, Steinmetz H, Seiler A, Lapa S, Steiner T, Thonke S, Weidauer S, Konczalla J, Hattingen E, Foerch C (2021) Multicenter prospective analysis of hypertrophic olivary degeneration following infratentorial stroke (HOD-IS): evaluation of disease epidemiology, clinical presentation, and MR-imaging aspects. Front Neurol 12:675123. https://doi.org/10.3389/fneur.2021.675123
Shinohara Y, Kinoshita T, Kinoshita F, Kaminou T, Watanabe T, Ogawa T (2013) Hypertrophic olivary degeneration after surgical resection of brain tumors. Acta Radiol 54:462–466. https://doi.org/10.1258/ar.2012.120537
Tartaglione T, Izzo G, Alexandre A, Botto A, Di Lella GM, Gaudino S, Caldarelli M, Colosimo C (2015) MRI findings of olivary degeneration after surgery for posterior fossa tumours in children: incidence, time course and correlation with tumour grading. Radiol Med 120:474–482. https://doi.org/10.1007/s11547-014-0477-x
Yagura H, Miyai I, Hatakenaka M, Yanagihara T (2007) Inferior olivary hypertrophy is associated with a lower functional state after pontine hemorrhage. Cerebrovasc Dis 24:369–374. https://doi.org/10.1159/000106984
Yun J-H, Ahn JS, Park JC, Kwon DH, Kwun BD, Kim CJ (2013) Hypertrophic olivary degeneration following surgical resection or gamma knife radiosurgery of brainstem cavernous malformations: an 11-case series and a review of literature. Acta Neurochir 155:469–476. https://doi.org/10.1007/s00701-012-1567-y
Kawata Y, Suzuki T, Kagaya H, Omi R, Shiroto H, Ebina K (1996) An MRI analysis of brain-stem and cerebellar lesions and olivary hypertrophy. Neuroradiology 38:441–443. https://doi.org/10.1007/bf00607269
Ruiz-Sandoval JL, Chiquete-Anaya E, López-Valencia G, González-Lara A, Andrade-Ramos MA, Macías-Reyes H, Jiménez-Ruiz A, Rosas-Razo B (2020) Symptomatic palatal tremor: A descriptive cohort study of 27 cases in a tertiary hospital. Revista Mexicana de Neurociencia 21(5):199–204. https://doi.org/10.24875/rmn.20000019
Schaller-Paule MA, Foerch C, Kluge S, Baumgarten P, Konczalla J, Steinbach JP, Wagner M, Luger A-L (2019) Delayed occurrence of hypertrophic olivary degeneration after therapy of posterior fossa tumors: a single institution retrospective analysis. J Clin Med 8(12):2222. https://doi.org/10.3390/jcm8122222
Uchino A, Hasuo K, Uchida K, Matsumoto S, Tsukamoto Y, Ohno M, Masuda K (1993) Olivary degeneration after cerebellar or brain stem haemorrhage: MRI. Neuroradiology 35:335–338. https://doi.org/10.1007/bf00588362
Matsuo F, Ajax ET (1979) Palatal myoclonus and denervation supersensitivity in the central nervous system. Ann Neurol 5:72–78. https://doi.org/10.1002/ana.410050111
Beylergil SB, Gupta P, Shaikh AG (2020) Does inferior-olive hypersynchrony affect vestibular heading perception? The Cerebellum 20:744–750. https://doi.org/10.1007/s12311-020-01103-z
Fukushima K, Mizuno Y, Takatama M, Okamoto K (2006) Increased neuronal expression of alpha B-crystallin in human olivary hypertrophy. Neuropathology 26:196–200. https://doi.org/10.1111/j.1440-1789.2006.00682.x
Kawanami T, Kato T, Llena JF, Hirano A, Sasaki H (1994) Altered synaptophysin-immunoreactive pattern in human olivary hypertrophy. Neurosci Lett 176:178–180. https://doi.org/10.1016/0304-3940(94)90076-0
Takamine K, Okamoto K, Fujita Y, Sakurai A, Takatama M, Gonatas NK (2000) The involvement of the neuronal Golgi apparatus and trans-Golgi network in the human olivary hypertrophy. J Neurol Sci 182:45–50. https://doi.org/10.1016/s0022-510x(00)00447-0
Kumar N, Eggers SDZ, Milone M, Keegan BM (2011) Acquired progressive ataxia and palatal tremor: importance of MRI evidence of hemosiderin deposition and vascular malformations. Parkinsonism Relat Disord 17:565–568. https://doi.org/10.1016/j.parkreldis.2011.04.018
Ogawa K, Kamei S, Ichihara K, Uehara K, Suzuki Y, Uchihara T, Yoshihashi H, Chong J-M (2014) Immunohistochemical study of pseudohypertrophy of the inferior olivary nucleus. Clin Neuropathol 33:68–75. https://doi.org/10.5414/np300594
Okamoto K, Hirai S, Lizuka T, Watanabe M (1992) Fundamental morphological changes in human olivary hypertrophy. Pathol Int 42:408–413. https://doi.org/10.1111/j.1440-1827.1992.tb03245.x
Ogawa K, Mizutani T, Uehara K, Minami M, Suzuki Y, Uchihara T (2010) Pathological study of pseudohypertrophy of the inferior olivary nucleus. Neuropathology 30:15–23. https://doi.org/10.1111/j.1440-1789.2009.01033.x
Nishie M, Yoshida Y, Hirata Y, Matsunaga M (2002) Generation of symptomatic palatal tremor is not correlated with inferior olivary hypertrophy. Brain 125:1348–1357. https://doi.org/10.1093/brain/awf126
Kesimal U, Karaali K, Senol U (2021) Radiological significance of symmetric central tegmental tract hyperintensity in pediatric patients. Iran J Radiol 18. https://doi.org/10.5812/iranjradiol.108100
Goto N, Kaneko M (1981) Olivary enlargement: chronological and morphometric analyses. Acta Neuropathol 54:275–282. https://doi.org/10.1007/bf00697000
Sarnat HB, Flores-Sarnat L, Auer RN (2013) Sequence of synaptogenesis in the fetal and neonatal cerebellar system - part 1: Guillain-Mollaret triangle (dentato-rubro-olivo-cerebellar circuit). Dev Neurosci 35:69–81. https://doi.org/10.1159/000350503
Madhavan A, Carr CM, Krecke KN, Wood CP, McKeon A, Dubey D (2021) Association between paraneoplastic rhombencephalitis and hypertrophic olivary degeneration. J Neurol Neurosurg Psychiatry 92:798–800. https://doi.org/10.1136/jnnp-2020-325569
Koga M, Tsutsumi A, Shirabe T (1997) The pathogenesis of olivary changes in clioquinol intoxication. Neuropathology 17:290–294. https://doi.org/10.1111/j.1440-1789.1997.tb00055.x
Nagappa M, Bindu PS, Sinha S, Bharath RD, Sandhya M, Saini J, Mathuranath PS, Taly AB (2017) Palatal tremor revisited: disorder with nosological diversity and etiological heterogeneity. Can J Neurol Sci 45:243–247. https://doi.org/10.1017/cjn.2017.273
Bulleid L, Hughes T, Leach P (2021) Mollaret's triangle: an important neuroanatomical territory for all clinicians. Surg Neurol Int 12:94. https://doi.org/10.25259/sni_625_2020
Patay Z, Enterkin J, Harreld JH, Yuan Y, Lobel U, Rumboldt Z, Khan R, Boop F (2013) MR Imaging evaluation of inferior olivary nuclei: comparison of postoperative subjects with and without posterior fossa syndrome. Am J Neuroradiol 35:797–802. https://doi.org/10.3174/ajnr.A3762
Yecies D, Jabarkheel R, Han M, Kim Y-H, Bruckert L, Shpanskaya K, Perez A, Edwards MSB, Grant GA, Yeom KW (2019) Posterior fossa syndrome and increased mean diffusivity in the olivary bodies. J Neurosurg Pediatr 24:376–381. https://doi.org/10.3171/2019.5.peds1964
Murdoch S, Shah P, Jampana R (2016) The Guillain–Mollaret triangle in action. Pract Neurol 16:243–246. https://doi.org/10.1136/practneurol-2015-001142
Zarranz Imirizaldu JJ (2014) Regarding the famous triangle of Guillain-Mollaret. Neurología 29:441–442. https://doi.org/10.1016/j.nrleng.2013.03.003
Mollaret M (1944) La meningite endothelio-leucotaire multirecurrent benigne: Syndrome nouveau ou maladie nouvelle? Rev Neurol 76:57–76
Moon SY, Cho SS, Kim YK, Kim SE, Kim JH, Kim JS (2007) Cerebral glucose metabolism in oculopalatal tremor. Eur J Neurol 15(1):42–49. https://doi.org/10.1111/j.1468-1331.2007.01997.x
Shaikh AG, Wong AL, Optican LM, Zee DS (2016) Impaired motor learning in a disorder of the inferior olive: is the cerebellum confused? The Cerebellum 16:158–167. https://doi.org/10.1007/s12311-016-0785-x
Lavezzi AM, Farronato G, Mauri M, Santoro F, Matturri L (2011) Common neurological alterations of the dentato-rubro-olivary pathway (Guillain-Mollaret Triangle) in different pathological fields: from palatal myoclonus to sudden unexplained fetal and infant death-a possible interpretation. Eur J Neurol 3:8–14
Raina GB, Cersosimo MG, Folgar SS, Giugni JC, Calandra C, Paviolo JP, Tkachuk VA, Zuñiga Ramirez C, Tschopp AL, Calvo DS, Pellene LA, Uribe Roca MC, Velez M, Giannaula RJ, Fernandez Pardal MM, Micheli FE (2016) Holmes tremor. Neurology 86:931–938. https://doi.org/10.1212/wnl.0000000000002440
Chang S-y, Kim Y, Park SJ (2020) Transsynaptic degeneration of the Guillain-Mollaret triangle after traumatic brain injury. Parkinsonism Relat Disord 78:9–11. https://doi.org/10.1016/j.parkreldis.2020.05.028
Choi S-M (2016) Movement disorders following cerebrovascular lesions in cerebellar circuits. J Mov Disord 9:80–88. https://doi.org/10.14802/jmd.16004
Liu Y, Gao B, Miao Y, Dong X (2020) Iron deposits in the red nucleus at long-term follow-up after hypertrophic olivary degeneration. Quant Imaging Med Surg 11:3371–3375
Schaller-Paule MA, Baumgarten P, Seifert V, Wagner M, Steidl E, Hattingen E, Wicke F, Steinbach JP, Foerch C, Konczalla J (2021) A paravermal trans-cerebellar approach to the posterior fossa tumor causes hypertrophic olivary degeneration by dentate nucleus injury. Cancers (Basel) 13:258. https://doi.org/10.3390/cancers13020258
Theeranaew W, Thurtell MJ, Loparo K, Shaikh AG (2020) Gabapentin and memantine increases randomness of oscillatory waveform in ocular palatal tremor. J Comput Neurosci 49:319–331. https://doi.org/10.1007/s10827-020-00753-6
Surisetti BK, Prasad S, Holla VV, Neeraja K, Kamble N, Netravathi M, Yadav R, Pal PK (2021) Clinical and imaging profile of patients with palatal tremor. Mov Disord Clin Pract 8:435–444. https://doi.org/10.1002/mdc3.13173
Author information
Authors and Affiliations
Contributions
EO: supervision, project development, data collection, manuscript writing. KA: data collection, manuscript writing. DT: data collection, figure illustration . The authors described their own experience, and all authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Ethics approval is not required for this review.
Consent to participate
Not applicable.
Consent for publication
All authors consent for publication.
Conflict of interest
The authors declare no competing interests.
Informed consent on studies with human and animal subjects
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ogut, E., Armagan, K. & Tufekci, D. The Guillain-Mollaret triangle: a key player in motor coordination and control with implications for neurological disorders. Neurosurg Rev 46, 181 (2023). https://doi.org/10.1007/s10143-023-02086-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10143-023-02086-1