Abstract
The aim of the study was to compare the different approaches of pre-operative diffusion-tensor-imaging-based fibre tracking (FT) of the corticospinal tract (CST) focusing on the positioning of the seeding region of interest (seed ROI). Thirty-nine patients with brain lesions in the vicinity of the CST were evaluated pre-operatively. Imaging comprised a 3D T1-weighted sequence, a gradient echo echo-planar imaging sequence for functional magnetic resonance imaging (fMRI), and a diffusion-weighted sequence for diffusion tensor (DT) tractography. DT tractography was performed with two different procedures to track the corticospinal fibres: one downwards and one upwards. Downward FT was started with the seed ROI in the pre-central gyrus subjacent to the maximal fMRI activity while for the upward FT seed ROI was placed in the cerebral peduncle. In 16 patients, tracking results were individually compared with the unaffected contralateral hemisphere. Results were correlated with fractional anisotropy (FA) values and other factors potentially influencing fibre tracking results. On the side with the space-occupying lesion, downward FT yielded more positive tracking results (tracked fibres > 0) than the upward FT. On both the affected and the unaffected side, downward FT reconstructed fewer fibres than upward FT. For none of the two methods did the tracking results (number and volume of fibres) correlate with FA values or with other clinical data. FA values for tracts ipsilateral to the lesion correlated with age and lesion entity. We conclude that the sequence of ROI positioning influences significantly the tracking results. Upward FT may fail to track fibres, whereas the successful tracking results may be superior to downward FT. Hence, upward FT of the CST should be preferred in patients with space-occupying lesions. Downward FT should be performed if upward FT fails.






Similar content being viewed by others
References
Bürgel U, Amunts K, Hoemke L, Mohlberg H, Gilsbach JM, Zilles K (2006) White matter fibre tracts of the human brain: three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage 29:1092–1105
Chen X, Weigel D, Ganslandt O, Fahlbusch R, Buchfelder M, Nimsky C (2007) Diffusion tensor-based fibre tracking and intraoperative neuronavigation for the resection of a brainstem cavernous angioma. Surg Neurol 68:285–291
Field AS, Alexander AL, Wu YC, Hasan KM, Witwer B, Badie B (2004) Diffusion tensor eigenvector directional color imaging patterns in the evaluation of cerebral white matter tracts altered by tumor. J Magn Reson Imaging 20:555–562
Heiervang E, Behrens TE, Mackay CE, Robson MD, Johansen-Berg H (2006) Between session reproducibility and between subject variability of diffusion MR and tractography measures. Neuroimage 33:867–77
Holodny AI, Ollenschleger MD, Liu WC, Schulder M, Kalnin AJ (2001) Identification of the corticospinal tracts achieved using blood-oxygen-level-dependent and diffusion functional MR imaging in patients with brain tumors. AJNR Am J Neuroradiol 22:83–88
Holodny AI, Gor DM, Watts R, Gutin PH, Ulug AM (2005) Diffusion-tensor MR tractography of somatotopic organization of corticospinal tracts in the internal capsule: initial anatomic results in contradistinction to prior reports. Radiology 234:649–653
Huang H, Zhang J, van Zijl PC, Mori S (2004) Analysis of noise effects on DTI-based tractography using the brute-force and multi-ROI approach. Magn Reson Med 52:559–565
Jellison BJ, Field AS, Medow J, Lazar M, Salamat MS, Alexander AL (2004) Diffusion tensor imaging of cerebral white matter: a pictorial review of physics, fibre tract anatomy, and tumor imaging patterns. AJNR Am J Neuroradiol 25:356–369
Jones DK, Simmons A, Williams SC, Horsfield MA (1999) Non-invasive assessment of axonal fibre connectivity in the human brain via diffusion tensor MRI. Magn Reson Med 42:37–41
Lazar M, Weinstein DM, Tsuruda JS, Hasan KM, Arfanakis K, Meyerand ME, Badie B, Rowley HA, Haughton V, Field A, Alexander AL (2003) White matter tractography using diffusion tensor deflection. Hum Brain Mapp 18:306–321
Lu S, Ahn D, Johnson G, Law M, Zagzag D, Grossman RI (2004) Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology 232:221–228
Mori S, van Zijl PC (2002) Fibre tracking: principles and strategies—a technical review. NMR Biomed 15:468–480
Nimsky C, Ganslandt O, Fahlbusch R (2006) Implementation of fibre tract navigation. Neurosurgery 58(4 Suppl 2):ONS-292–303
Nusbaum AO, Tang CY, Buchsbaum MS, Wei TC, Atlas SW (2001) Regional and global changes in cerebral diffusion with normal aging. Am J Neuroradiol 22:136–142
Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M (2000) Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med 44:259–268
Provenzale JM, McGraw P, Mhatre P, Guo AC, Delong D (2004) Peritumoral brain regions in gliomas and meningiomas: investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology 232:451–460
Schonberg T, Pianka P, Hendler T, Pasternak O, Assaf Y (2006) Characterization of displaced white matter by brain tumors using combined DTI and fMRI. Neuroimage 30:1100–1111
Smits M, Vernooij MW, Wielopolski PA, Vincent AJPE, Houston GC, van der Lugt A (2007) Incorporating functional MR imaging into diffusion tensor tractography in the preoperative assessment of the corticospinal tract in patients with brain tumors. AJNR Am J Neuroradiol 28:1354–1361
Weinstein DM, Kindlman GL, Lundberg EC (1999) Tensorlines: advection diffusion based propagation through diffusion tensor fields. In: Ebert DS, Gross MH, Hamann B (eds) IEEE visualization 1999. IEEE Computer Society and ACM, San Francisco, pp 249–253
Westin CF, Maier SE, Khidhir B, Everett P, Jolesz FA, Kikinis R (1999) Image processing for diffusion tensor magnetic resonance imaging. In: Taylor C, Colchester A (eds) Proceedings of the Second International Conference on Medical Image Computing and Computer-Assisted Interventions (MICCAI’99). Cambridge, Springer, pp 441–452
Yamada K, Kizu O, Mori S, Ito H, Nakamura H, Yuen S, Kubota T, Tanaka O, Akada W, Sasajima H, Mineura K, Nishimura T (2003) Brain fibre tracking with clinically feasible diffusion-tensor MR imaging: initial experience. Radiology 227:295–301
Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, Winkler P (1997) Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain 120:141–157
Zaitsev M, Hennig J, Speck O (2004) Point spread function mapping with parallel imaging techniques and high acceleration factors: fast, robust, and flexible method for echo-planar imaging distortion correction. Magn Reson Med 52:1156–1166
Conflict of interest Statement
The authors have no conflicts of interest in the subject matter of their manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Christopher Nimsky, Marburg, Germany
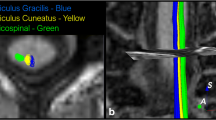
Diffusion tensor imaging (DTI) is increasingly applied in pre-operative neurosurgical evaluation to delineate the course of major white matter tracts, mostly the pyramidal tract. This information is then used intraoperatively to localise the white matter tract of interest to preserve neurological function.
Standard DTI is probably no longer the most elegant method to reconstruct major white matter tracts because, besides of spatial distortions of the raw data, which cause some inaccuracies, when these data are used in a navigation/stereotactic setup, there are some distinct challenges and inconsistencies when tracking algorithms are applied. Areas of white matter tract crossings cannot be resolved reliably by standard DTI; also, tracking that is initiated at different areas/volumes in the three-dimensional diffusion data set does not result in the identical anatomical structure. Ideally, a tracking algorithm strategy that starts in a part of the pyramidal tract should be able to reconstruct the pyramidal tract independent of the placement of the seed region.
The paper by Hattingen et al. highlights these problems by analysing the effects of seed region placement when applying a standard DTI tracking approach. It clearly demonstrates that distinct strategies on how to perform a multi-step tracking process have to be established. This is also of special importance, when the data are to be used for comparisons in larger patient groups. Furthermore, it has to be emphasised that a profound knowledge of how the original diffusion data are measured, what kind of sequences are used, and what kind of tracking algorithms are implemented and how they are optimally used is necessary if tractography is used in a clinical setting. Otherwise, tractography might not increase the safety for the neurosurgeon but result in increased risks for the patients.
Michael Nelles, Horst Urbach, Bonn, Germany
The combination of pre-surgical MRI as one of the most common examinations in clinical routine and diffusion tensor tractography (DTT) imposes the task of registering EPI-based sequences to “conventional” sequences for anatomic reference. The combination of fMRI with DTT yields additional information concerning a starting step for DTT in the primary motor area. Multi-step tracking can reduce unwanted tract contributions when the general trajectory of the tract of interest is known.
Size and location of the seeding ROIs affect, however, amongst different other factors, tractography results with respect to which of the displayed trajectories can be regarded as “true positive” or “negative”. Systematic evaluations of these factors and their inferences on fibre tracking results are still rare. The above-mentioned EPI-related artefacts (as well as patient motion) may lead to further erroneously “small” calculation of CST fibre bundles. An unguided placement of ROIs is prone to errors which adds to the general inter-observer variability of ROI-based DTT post-processing.
This study compares different approaches of fibre tracking of the CST in patients with intra-cranial mass lesions in the vicinity of the CST. A relatively thin-sectioned 1.6 × 1.6 × 1.6-mm isotropic sequence with whole-brain coverage and 12 encoding directions is used. Rigid registration fusion with high-resolution T1-weighted anatomical data and fMRI is used to allow for a guided placement of tracking seeds.
A particular focus is laid on the positioning of these seeding ROIs, as well as determination of the intra- and inter-rater variability of the tracking protocol. Systematic comparison of CST tractographies between affected and unaffected hemispheres contributes to the detection of fibre reduction due to technical issues (and thus missing fibre visualisation in a stereotactic setting). This paper hence provides essential data to improve the accuracy of pre-surgical DTT in terms of reproducibility and precision.
Elke Hattingen and Julian Rathert contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hattingen, E., Rathert, J., Jurcoane, A. et al. A standardised evaluation of pre-surgical imaging of the corticospinal tract: where to place the seed ROI. Neurosurg Rev 32, 445–456 (2009). https://doi.org/10.1007/s10143-009-0197-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-009-0197-1