Abstract
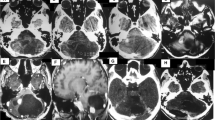
A retrospective study on solid central nervous system haemangioblastomas was performed to characterize clinical features, treatment strategies and outcome in these rare lesions. Between 1993 and 2006 23, solid haemangioblastomas were surgically removed in 17 patients. Eight lesions were located within pons Varolii and medulla oblongata, six within the cerebellar hemispheres and three in the cerebellopontine angle. Three haemangioblastomas were located supratentorially and three within the spinal cord. All patients except two underwent pre-operative magnetic resonance imaging (MRI). Post-operative digital subtraction angiography and/or MRI was performed in all surviving patients. Except for spinal cord lesions, rather unsystematic clinical symptoms were observed. Twenty-two tumours could be resected completely. Two patients with brainstem lesions died within 10 weeks after surgery from infectious complications. Persistent new neurological deficits occurred in two patients. Three patients underwent radiosurgery prior to or following the surgical procedure. Solid haemangioblastomas represent a surgical challenge due to their arteriovenous malformation-like vascularisation and their frequent location in eloquent areas. Surgery is the therapy of choice. Circumferential dissection with devascularization and en bloc removal yields good functional results. A location within the brainstem carries the most unfavourable prognosis.







Similar content being viewed by others
References
Biondi A, Ricciardi GK, Faillot T, Capelle L, Van Effenterre R, Chiras J (2005) Hemangioblastomas of the lower spinal region: report of four cases with preoperative embolization and review of the literature. Am J Neuroradiol 26:936–945
Chang SD, Meisel JA, Hancock SL, Martin DP, Mc Manus M, Adler JR Jr (1998) Treatment of hemangioblastomas in von Hippel–Lindau disease with linear-accelerator-based radiosurgery. Neurosurgery 43:28–35
Chen F, Kishida T, Yao M, Hustad T, Glavac D, Dean M, Gnarra JR (1995) Germline mutations in the von Hippel–Lindau disease tumor suppressor gene: correlations with phenotype. Hum Mutat 5:66–75
Chen F, Slife, Kishida T, Mulvihill J, Tishermann SE, Zbar B (1996) Genotype-phenotype correlation in von Hippel–Lindau disease: identification of a mutation associated with VHL type 2A. J Med Genet 33:716–717
Chou SN, Erickson DL, Ortiz-Suarez JH (1975) Surgical treatment of vascular lesions in the brain stem. J Neurosurg 42:23–31
Conway JE, Chou D, Clatterbuck RE, Brem H, Long DM, Rigamonti D (2001) Hemangioblastomas of the central nervous system in von Hippel–Lindau syndrome and sporadic disease. Neurosurgery 48(1):55–62
Cornelius JF, Saint-Maurice JP, Bresson D, George B, Houdart E (2007) Hemorrhage after particle embolization of hemangioblastomas: comparison of outcomes in spinal and cerebellar lesions. J Neurosurg 106:994–998
Dow GR, Sim DW, O’Sullivan MG (2002) Excision of large solid haemangioblastomas of the cerebellopontine angle by a skull base approach. Br J Neurosurg 16(2):168–171
Eskridge JM, McAuliffe W, Harris B, Kim DK, Scott J, Winn HR (1996) Preoperative endovascular embolization of craniospinal hemangioblastomas. Am J Neuroradiol 17(3):525–531
Filling-Katz MR, Choyke PL, Oldfield E, Charnas L, Patronas NJ, Glenn GM, Gorin MB, Morgan JK, Linehan WM, Seizinger BR (1991) Central nervous system involvement in von Hippel–Lindau disease. Neurology 41:41–46
Glenn GM, Linehan WM, Hosoe S, Latif F, Yao M, Choyke P, Gorin MB et al (1992) Screening for von Hippel–Lindau disease by DNA polymorphism analysis. JAMA 267:1226–1231
Jawahar A, Kondziolka D, Garces YI, Flickinger JC, Pollock BE, Lunsford LD (2000) Stereotactic radiosurgery for hemangioblastomas of the brain. Acta Neurochir (Wien) 142(6):641–644
Julow J, Balint K, Gortvai P, Pasztor E (1994) Posterior fossa haemangioblastomas. Acta Neurochir (Wien) 128(1–4):109–114
Ho YS, Plets C, Goffin J, Dom R (1990) Hemangioblastoma of the lateral ventricle. Surg Neurol 33:407–412
Hosoe S, Brauch H, Latif F, Glenn G, Daniel F, Bale S, Choyke P et al (1990) Localization of the von Hippel–Lindau disease gene to a small region of chromosome 3. Genomics 8:634–640
Krishnan KG, Schackert G (2006) Outcomes of surgical resection of large solitary hemangioblastomas of the craniocervical junction with limitations in preoperative angiographic intervention: report of three cases. Zentralbl Neurochir 67:137–143
Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, Stackhouse T et al (1993) Identification of the von Hippel–Lindau disease tumor suppressor gene. Science 260:1317–1320
Lonser R, Weil R, Wanebo J, DeVroom H, Oldfield E (2003) Surgical management of spinal cord hemangioblastomas in patients with von Hippel–Lindau disease. J Neurosurg 98:106–116
Machein MR, Plate KH (2000) VEGF in brain tumors. J Neurooncol 50(1–2):109–120
Mans DA, Lolkema MP, van Beest M, Daenen LG, Voest EE, Giles RH (2008) Mobility of the von Hippel–Lindau tumour suppressor protein is regulated by kinesin-2. Exp Cell Res 14:1229–1236
Naik RTS, Purohit AK, Dinakar I, Ratnakar KS (1995) Hemangioblastoma of the IV. ventricle. Childs Nerv Syst 11:499–500
Neumann HP, Wiestler OD (1994) Von Hippel–Lindau disease: a syndrome providing insights into growth control and tumorigenesis. Nephrol Dial Transplant 9:1832–1833
Niemela M, Lim YJ, Soderman M, Jääskeläinen J, Lindquist C (1996) Gamma knife radiosurgery in 11 hemangioblastomas. J Neurosurg 85:591–596
Okawara SH (1973) Solid cerebellar hemangioblastoma. J Neurosurg 39:514–518
Palmer JJ (1972) Hemangioblastomas. A review of 81 cases. Acta Neurochir 27:125–148
Pan L, Wang EM, Wang BJ, Zhou LF, Zhan N, Cai PW, Da JZ (1998) Gamma knife radiosurgery for hemangioblastomas. Stereotact Funct Neurosurg 70(Suppl):179–186
Rawe SE, Van Gilder JC, Rothman SLG (1978) Radiographic diagnostic evaluation and surgical treatment of multiple cerebellar, brain stem, and spinal cord hemangioblastomas. Surg Neurol 9:337–341
Resche F, Moisan JP, Mantoura J, De Kersaint-Gilly A, Andre MJ, Perrin-Resche I, Menegalli-Bogelli D, Lajat Y, Richard S (1993) Haemangioblastoma, haemangioblastomatosis, and von Hippel–Lindau disease. Adv Technol Stand Neurosurg 20:197–304
Rodesch G, Gaillard S, Loiseau H, Brotchi J (2008) Embolization of intradural vascular spinal cord tumors: Report of five cases and review of the literature. Neuroradiology 50:145–151
Russel DS, Rubinstein WLJ (eds) (1989) Pathology of tumors of the nervous system. Arnold, 5th edn, London
Ryu SI, Kim DH, Chang SD (2003) Stereotactic radiosurgery for hemangiomas and ependymomas of the spinal cord. Neurosurg Focus 15(5):E10
Sanford RA, Smith RA (1986) Haemangioblastomas of the cervico-medullary junction. J Neurosurg 64:317–321
Santarpia L, Sarlis NJ, Santarpia M, Sherman SI, Trimarchi F, Benvenga S (2007) Mosaicism in von Hippel–Lindau disease: an event important to recognize. J Cell Mol Med 11:1408–1415
Seizinger BR, Rouleau GA, Ozelius LJ, Lane AH, Farmer GE, Lamiell JM, Haines J et al (1988) Von Hippel–Lindau disease maps to the region of chromosome 3 associated with renal cell carcinoma. Nature 332:268–269
Singounas EG (1978) Hemangioblastomas of the central nervous system. Acta Neurochir (Wien) 44:107–113
Spetzger U, Bertalanffy H, Huffmann B, Mayfrank L, Reul J, Gilsbach JM (1996) Hemangioblastomas of the spinal cord and the brainstem: diagnostic and therapeutic features. Neurosurg Rev 19:147–151
Symon L, Murota T, Pell M, Bordi L (1993) Surgical management of haemangioblastoma of the posterior fossa. Acta Neurochir (Wien) 120(3–4):103–110
Tago M, Terahara A, Shin M, Maruyama K, Kurita H, Nakagawa K, Ohtomo K (2005) Gamma knife surgery for hemangioblastomas. J Neurosurg 102(Suppl):171–174
Takeuchi S, Tanaka R, Fujii Y, Abe H, Ito Y (2001) Surgical treatment of hemangioblastomas with presurgical endovascular embolization. Neurol Med Chir (Tokyo) 41(5):246–251
Tampieri D, Leblanc R, TerBrugge K (1993) Preoperative embolization of brain and spinal hemangioblastomas. Neurosurgery 33(3):502–505
Van Velthoven V, Reinacher PC, Klisch J, Neumann HP, Glasker S (2003) Treatment of intramedullary hemangioblastomas, with special attention to von Hippel–Lindau disease. Neurosurgery 53(6):1306–1313
Vazquez-Anon V, Botella C, Beltran A, Solera M, Piquer J (1997) Preoperative embolization of solid cervicomedullary junction hemangioblastomas: report of two cases. Neuroradiology 39(2):86–89
Wanebo JE, Lonser R, Glenn G, Oldfield E (2003) The natural history of hemangioblastomas of the central nervous system in patients with von Hippel–Lindau disease. J Neurosurg 98:82–94
Wang C, Zhang J, Liu A, Sun B (2001) Surgical management of medullary hemangioblastoma. Report of 47 cases. Surg Neurol 56(4):218–226
Wang EM, Pan L, Wang BJ, Zhang N, Zhou LF, Dong YF, Dai JZ, Cai PW, Chen H (2005) The long-term results of gamma knife radiosurgery for hemangioblastomas of the brain. J Neurosurg 102(Suppl):225–229
Weil RJ, Lonser RR, DeVroom HL, Wanebo JE, Oldfield EH (2003) Surgical management of brainstem hemangioblastomas in patients with von Hippel–Lindau disease. J Neurosurg 98(1):95–105
Yasargil MG, Antic J, Laciga R, De Preux J, Fideler RW, Boone SC (1976) The microsurgical removal of intramedullary spinal hemangioblastomas: Report of twelve cases and a review of the literature. Surg Neurol 6:141–146
Young S, Richardson AE (1987) Solid hemangioblastomas of the posterior fossa: radiological features and results of surgery. J Neurol Neurosurg Psychiatry 50:155–158
Zbar B, Kishida T, Chen F, Schmidt L, Maher ER, Richards FM, Crossey PA et al (1996) Germline mutations in the Von Hippel–Lindau disease (VHL) gene in families from North America, Europe, and Japan. Hum Mutat 8:348–357
Zimmermann M, Seifert V, Schreyer V, Stolke D, Dietz H (1997) Hemangioblastoma: Description of a disease picture and report of 41 cases. Zentralbl Neurochir 58(1):1–6
Zhou LF, Du G, Mao Y, Zhang R (2005) Diagnosis and surgical treatment of brainstem hemangioblastomas. Surg Neurol 63(4):307–315
Author information
Authors and Affiliations
Corresponding author
Additional information
Comments
Kartik G. Krishnan, Gabriele Schackert, Dresden, Germany
In this report on solid hemangioblastomas of the central nervous system, Rachinger et al. analyze the outcomes after the surgical treatment of the condition in 17 patients (in all, 23 hemangioblastomas). The majority of the lesions (17 of 23) were found in the posterior cranial fossa, whereas three were located supratentorially and the rest three within the spinal cord.
Although hemangioblastomas are benign lesions, as per the histological description, major factors influencing outcomes of treatment are size and localization of the lesions. Hemangioblastomas preferentially tend to occur in the posterior cranial fossa. Here, as in the case of any other lesion, pons Varolii and brainstem are vulnerable regions.
Whereas small tumors in resectable locations can be completely excised, large tumors require a combination treatment strategy of interventional embolization and surgery. Pre-operative embolization of large hemangioblastomas of the posterior fossa helps to minimize intraoperative blood loss by virtue of reducing the vascularity of the tumors. Having said the above, even super-selective embolization of large hemangioblastomas of the craniocervical junction is not without infarction hazards, which may eventually turn life threatening. Thus, pre-operative embolization of large tumors that draw their vascularity from the magistrals of the posterior circulation is not always redeemable. Surgical resection is nevertheless indicated, owing to the space-occupying nature of the lesions, before bleeding or decompensation of the brain stem can occur. A favorable prognosis may be expected in these cases in the long run.
Uwe Spetzger, Karlsruhe, Germany
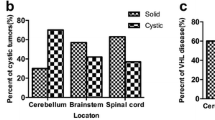
The authors present an interesting retrospective study to evaluate the clinical features and the therapeutic strategies in 17 patients, totally harbouring 23 solid haemangioblastomas of the CNS. Usually, haemangioblastomas appear cystic, solid with cystic components or solid. This study indicates that there might be differences in clinical manifestation between cystic and solid lesions. The mechanism for neurological deterioration is the growing of the lesion, especially the expansion of the cyst with focal compression of the surrounding tissue. Another potential problem could be a bleeding from a haemangioblastoma; this rare event increase morbidity and mortality significantly. However, this is a very unlikely event and is associated mainly with bigger lesions. There are no figures from the literature if solid haemangioblastomas have a higher rate of spontaneous bleeding, which is also not answered by this report. Considerably, small solid haemangioblastomas are in an early stage of their evolution, which eventually may transform into cystic ones. However, due to our own experience, we have also seen the transformation of cystic lesions into solid tumours.
The typical infratentorial location of haemangioblastomas within the brainstem or in the spinal cord is a challenge even for an experienced neurosurgeon. There is evidence that operative treatment of solid haemangioblastomas is more complex than that of cystic ones because the amount of the tissue and especially the vascular architecture and the blood supply of a solid component are much more complex to handle as the simple microsurgical removal of a cystic compartment.
For my experience, mainly the size and the location, respectively the access and the optimal visualization of a haemangioblastoma, are the decisive features to predict the surgical risk. The adequate pre-operative imaging allowing the exact localization of the arterial feeders and the venous drainage, as well as the intraoperative discovery and confirmation of those vessels, are the essentials for a well-ordered microsurgical removal, especially in huge tumours. Therefore, the whole armamentarium of vascular microsurgery including intraoperative blood-flow measurement, neuronavigation, electrophysiological monitoring and an experienced neuroanaesthesiologist are mandatory to reduce surgical morbidity.
The meticulously recorded results of the authors correspond to our own experiences; if the size of the haemangiobalstoma and the approach to the lesion allow an en bloc removal, usually, the post-operative results are favourable. These results are the basis to discuss the indication of non-surgical treatment modalities, especially in the evolving field of stereotactic radiosurgery.
Yoko Kato, Toyoake, Japan
The removal of a solid haemangioblastoma in an eloquent location remains one of the most formidable tasks even for an experienced neurosurgeon; even in the best hands, some of these lesions are to be left for other modalities of treatment. The meticulously recorded experience of the authors is valuable for decision making in these difficult-to-treat tumors; however, the variability of locations (spinal cord, brain stem, cerebellar hemispheres and supratentorial lesions), makes it difficult to draw conclusive evidence.
For haemangioblastoma being solid is considered to be an early stage in their evolution, which eventually may transform into a cystic one, and that is true particularly for the small ones. If they are multiple (vHL disease), it is difficult to decide which one is symptomatic, and the expected morbidity if removed is the most important limiting factor in the decision.
In our experience, all brain stem lesions potentially may show post-operative lower cranial nerve deficits and have to be taken out of ventillatory support carefully, over the next 2–3 days, irrespective of the uncomplicated surgery.
A problematic event is a bleeding haemangioblastoma, an event that significantly increases morbidity, and life-saving emergency surgery should be considered; fortunately, the review of statistics showed that hemorrhage is a very unlikely event and is associated mainly with bigger lesions.
Removal “in toto” has been also our strategy, and we have abstained from embolizing brain stem lesions, avoiding the risk of complications.
With the increasing experience in stereotactic radiosurgery, we expect that the dividing line of indications between modalities will move towards less aggressive surgery.
Many important questions remain unanswered, particularly regarding the effect of cerebral blood flow changes in critical zones at the time of removal. The proper preservation of important arterial supply and venous drainage, the use of combined modalities of treatment and certainly a broader accumulation and evaluation of data is very important.
Rights and permissions
About this article
Cite this article
Rachinger, J., Buslei, R., Prell, J. et al. Solid haemangioblastomas of the CNS: a review of 17 consecutive cases. Neurosurg Rev 32, 37–48 (2009). https://doi.org/10.1007/s10143-008-0166-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10143-008-0166-0