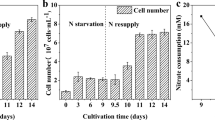
Abstract
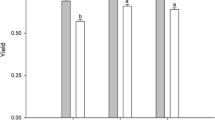
The effect of nitrogen and sulphur limitation under high irradiance (PAR) was studied in the green microalga Chlorella fusca (Chlorophyta) in order to follow lipid and/or starch accumulation. Growth, biomass composition and the changes in photosynthetic activity (in vivo chlorophyll a fluorescence) were followed in the trials. The full nutrient culture showed high biomass production and starch accumulation at Day 1, when photosynthetic activity was high. Gradual deprivation (no nutrients added) became evident when photosynthesis was significantly suppressed (Day 3 onwards), which entailed a decrease of maximum relative electron transport rate (rETRmax) and increase of non-photochemical quenching (NPQ), accompanied by the onset of lipid accumulation and decline in starch content. In N- and S-starved cultures, rETRmax significantly decreased by Day 3, which caused a substantial drop in biomass production, cell number, biovolume and induction of lipid and starch accumulation. High starch content (45–50 % of DW) was found at the initial stage in full nutrient culture and at the stationary phase in nutrient-starved cultures. By the end of the trial, all treatments showed high lipid content (~30 % of DW). The full nutrient culture had higher biomass yield than starved treatments although starch (~0.2 g L−1 day−1) and lipid (~0.15 g L−1 day−1) productivities were fairly similar in all the cultures. Our results showed that we could enrich biomass of C. fusca (% DW) in lipids using a two-stage strategy (a nutrient replete stage followed by gradual nutrient limitation) while under either procedure, N- or S-starvation, both high lipid and starch contents could be achieved.








Similar content being viewed by others
References
Adarme-Vega T, Lim DKY, Timmins M et al (2012) Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production. Microb Cell Factories 11:96
Allen MM, Stanier RY (1968) Growth and division of some unicellular blue-green algae. J Gen Microbiol 51:199–202
Araus JL, Amaro T, Voltas J et al (1998) Chlorophyll fluorescence as a selection criterion for grain yield in durum wheat under Mediterranean conditions. Field Crop Res 55:209–223
Berges JA, Falkowski PG (1998) Physiological stress and cell death in marine phytoplankton: induction of proteases in response to nitrogen or light limitation. Limnol Oceanogr 43:129–135
Berges JA, Charlebois DO, Mauzerall DC, Falkowski PG (1996) Differential effects of nitrogen limitation on photosynthetic efficiency of photosystems I and II in microalgae. Plant Physiol 110:689–696
Bilger W, Björkman O (1990) Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res 25:173–185
Bischof K, Gómez I, Molis M et al (2006) Ultraviolet radiation shapes seaweed communities. Rev Environ Sci Biotechnol 5:141–166
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Boussiba S (2000) Carotenogenesis in the green alga, Haematococcus pluvialis: cellular physiology and stress response. Physiol Plant 108:111–117
Brányiková I, Maršálková B, Doucha J et al (2011) Microalgae-novel highly efficient starch producers. Biotechnol Bioeng 108:766–776
Cakmak T, Angun P, Demiray YE et al (2012) Differential effects of nitrogen and sulfur deprivation on growth and biodiesel feedstock production of Chlamydomonas reinhardtii. Biotechnol Bioeng 109:1947–1957
Carfagna S, Salbitani G, Vona V, Esposito S (2011) Changes in cysteine and O-acetyl-l-serine levels in the microalga Chlorella sorokiniana in response to the S-nutritional status. J Plant Physiol 168:2188–2195
Carvalho AP, Malcata FX (2005) Optimization of omega-3 fatty acid production by microalgae: crossover effects of CO2 and light intensity under batch and continuous cultivation modes. Mar Biotechnol 7:381–388
Converti A, Casazza AA, Ortiz EY et al (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process Process Intensif 48:1146–1151
De Pauw N, Persoone G (1988) Microalgae for aquaculture. In: Borowitzka MA, Borowitzka LJ (eds) Micro-algal Biotechnology. Cambridge University Press, Cambridge, pp 197–221
Dean AP, Sigee DC, Estrada B, Pittman JK (2010) Using FTIR spectroscopy for rapid determination of lipid accumulation in response to nitrogen limitation in freshwater microalgae. Bioresour Technol 101:4499–4507
Demirbas MF (2010) Microalgae as a feedstock for biodiesel. Energy Educ Sci Technol Part A 25:31–43
Di Martino Rigano V, Vona V, Carfagna S, et al (2000) Effects of sulfate-starvation and re-supply on growth, NH4 + uptake and starch metabolism in Chlorella sorokiniana. Funct Plant Biol 27:335–342
Dragone G, Fernandes BD, Abreu AP et al (2011) Nutrient limitation as a strategy for increasing starch accumulation in microalgae. Appl Energy 88:3331–3335
Eilers PHC, Peeters JCH (1988) A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol Model 42:199–215
Fernandes B, Teixeira J, Dragone G et al (2013) Relationship between starch and lipid accumulation induced by nutrient depletion and replenishment in the microalga Parachlorella kessleri. Bioresour Technol 144:268–274
Figueroa F, Conde-Álvarez R, Bonomi Barufi J, Celis-Plá P, Flores P, Malta E, Stengel D, Meyerhoff O, Pérez-Ruzafa A (2014) Continuous monitoring of in vivo chlorophyll a fluorescence in Ulva rigida (Chlorophyta) submitted to different CO2, nutrient and temperature regimes. Aquat Biol 22:195–212
Figueroa FL, Mercado J, Jiménez C et al (1997) Relationship between bio-optical characteristics and photoinhibition of phytoplankton. Aquat Bot 59:237–251
Figueroa FL, Jerez CG, Korbee N (2013) Use of in vivo chlorophyll fluorescence to estimate photosynthetic activity and biomass productivity in microalgae grown in different culture systems. Lat Am J Aquat Res 41:801–819
Friedman O, Dubinsky Z, Arad Malis S (1991) Effect of light intensity on growth and polysaccharide production in red and blue-green rhodophyta unicells. Bioresour Technol 38:105–110
Geider RJ, MacIntyre HL, Graziano LM, McKay RML (1998) Responses of the photosynthetic apparatus of Dunaliella tertiolecta (Chlorophyceae) to nitrogen and phosphorus limitation. Eur J Phycol 33:315–332
Genty B, Harbinson J, Cailly AL, Rizza F (1996) Fate of excitation at PSII in leaves. The non-photochemical side. Plant Physiol Biochem 86 (Special Issue)
Giordano M, Pezzoni V, Hell R (2000) Strategies for the allocation of resources under sulfur limitation in the green alga Dunaliella salina. Plant Physiol 124:857–864
Griffiths MJ, Harrison STL (2009) Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J Appl Phycol 21:493–507
Griffiths MJ, Van Hille RP, Harrison STL (2014a) The effect of nitrogen limitation on lipid productivity and cell composition in Chlorella vulgaris. Appl Microbiol Biotechnol 98:2345–2356
Griffiths MJ, Van Hille RP, Harrison STL (2014b) The effect of degree and timing of nitrogen limitation on lipid productivity in Chlorella vulgaris. Appl Microbiol Biotechnol 98:6147–6159
Hartig P, Wolfstein K, Lippemeier S, Colijn F (1998) Photosynthetic activity of natural microphytobenthos populations measured by fluorescence (PAM) and 14C-tracer methods: a comparison. Mar Ecol Prog Ser 166:53–62
Ho SH, Chen CY, Chang JS (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalga Scenedesmus obliquus CNW-N. Bioresour Technol 113:244–252
Hocaoglu SM, Insel G, Cokgor EU et al (2010) COD fractionation and biodegradation kinetics of segregated domestic wastewater: black and grey water fractions. J Chem Technol Biotechnol 85:1241–1249
Hu Q (2003) Environmental effects on cell composition. In: A Richmond (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell Publishing Ltd, Oxford, pp 83–94
Jerez CG, Navarro E, Malpartida I et al (2014) Hydrodynamics and photosynthesis performance of Chlorella fusca grown in a thin-layer cascade (TLC) system. Aquat Biol 22:111–122
Jiang Y, Yoshida T, Quigg A (2012) Photosynthetic performance, lipid production and biomass composition in response to nitrogen limitation in marine microalgae. Plant Physiol Biochem 54:70–77
Juneau P, Green BR, Harrison PJ (2005) Simulation of Pulse-Amplitude-Modulated (PAM) fluorescence: limitations of some PAM-parameters in studying environmental stress effects. Photosynthetica 43:75–83
Klok AJ, Martens DE, Wijffels RH, Lamers PP (2013) Simultaneous growth and neutral lipid accumulation in microalgae. Bioresour Technol 134:233–243
Klughammer C, Schreiber U (2008) Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAN 1:27–35
Kromkamp JC, Forster RM (2003) The use of variable fluorescence measurements in aquatic ecosystems: differences between multiple and single turnover measuring protocols and suggested terminology. Eur J Phycol 38:103–112
Kromkamp JC, Dijkman NA, Peene J et al (2008) Estimating phytoplankton primary production in Lake IJsselmeer (The Netherlands) using variable fluorescence (PAM-FRRF) and C-uptake techniques. Eur J Phycol 43:327–344
Li Y, Horsman M, Wang B et al (2008) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Li Y, Han D, Hu G et al (2010) Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii. Biotechnol Bioeng 107:258–268
Li Y, Han D, Sommerfeld M, Hu Q (2011) Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour Technol 102:123–129
Li X, Přibyl P, Bišová K et al (2013) The microalga Parachlorella kessleri—a novel highly efficient lipid producer. Biotechnol Bioeng 110:97–107
Malapascua J, Jerez CG, Sergejevová M, et al. (2014) Photosynthesis monitoring to optimize growth of microalgal mass cultures: application of chlorophyll fluorescence techniques. Aquat Biol 22:123–140
Markou G, Nerantzis E (2013) Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol Adv 31:1532–1542
Masojídek J, Torzillo G, Kopecký J et al (2000) Changes in chlorophyll fluorescence quenching and pigment composition in the green alga, Chlorococcum sp. grown under nitrogen deficiency and salinity stress. J Appl Phycol 12:417–426
Masojídek J, Vonshak A, Torzillo G (2010) Chlorophyll fluorescence applications in microalgal mass cultures. In: Suggett DJ, Prášil O, Borowitzka MA (eds) Chlorophyll a fluorescence in aquatic sciences: methods and applications SE - 13. Springer-Dordrecht, The Netherlands, pp 277–292
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
McCready R, Guggolz J, Silviera V, Owens H (1950) Determination of starch and amylose in vegetables. Anal Chem 22:1156–1158
Menon KR, Balan R, Suraishkumar GK (2013) Stress induced lipid production in Chlorella vulgaris: relationship with specific intracellular reactive species levels. Biotechnol Bioeng 110:1627–1636
Mizuno Y, Sato A, Watanabe K et al (2013) Sequential accumulation of starch and lipid induced by sulfur deficiency in Chlorella and Parachlorella species. Bioresour Technol 129:150–155
Mujtaba G, Choi W, Lee CG, Lee K (2012) Lipid production by Chlorella vulgaris after a shift from nutrient-rich to nitrogen starvation conditions. Bioresour Technol 123:279–283
Pires JCM, Alvim-Ferraz MCM, Martins FG, Simões M (2012) Carbon dioxide capture from flue gases using microalgae: engineering aspects and biorefinery concept. Renew Sustain Energy Rev 16:3043–3053
Praveenkumar R, Shameera K, Mahalakshmi G et al (2012) Influence of nutrient deprivations on lipid accumulation in a dominant indigenous microalga Chlorella sp., BUM11008: evaluation for biodiesel production. Biomass Bioenergy 37:60–66
Přibyl P, Cepák V, Zachleder V (2012) Production of lipids in 10 strains of Chlorella and Parachlorella and enhanced lipid productivity in Chlorella vulgaris. Appl Microbiol Biotechnol 94:549–561
Procházková G, Brányiková I, Zachleder V, Brányik T (2014) Effect of nutrient supply status on biomass composition of eukaryotic green microalgae. J Appl Phycol 26:1359–1377
Rippka R, Deruelles J, Waterbury JB et al (1979) Generic assignments, strain histories and properties of pure cultures of Cyanobacteria. J Gen Microbiol 111:1–61
Rodolfi L, Zittelli GC, Bassi N et al (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Schreiber U, Müller JF, Haugg A, Gademann R (2002) New type of dual-channel PAM chlorophyll fluorometer for highly sensitive water toxicity biotests. Photosynth Res 74:317–330
Sharma KK, Schuhmann H, Schenk PM (2012) High lipid induction in microalgae for biodiesel production. Energies 5:1532–1553
Sirisansaneeyakul S, Singhasuwan S, Choorit W et al (2011) Photoautotrophic production of lipids by some Chlorella strains. Mar Biotechnol 13:928–941
Solovchenko AE, Khozin-Goldberg I, Didi-Cohen S et al (2008) Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J Appl Phycol 20:245–251
Stephenson AL, Dennis JS, Howe CJ et al (2010) Influence of nitrogen-limitation regime on the production by Chlorella vulgaris of lipids for biodiesel feedstocks. Biofuels 1:47–58
Strasser R, Srivastava A, Tsimilli-Michael M (2000) The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Yunus M, Pathre U, Mohanty P (eds) Probing photosynthesis: mechanism, regulation and adaptation. Taylor & Francis, London, pp 445–483
Su CH, Chien LJ, Gomes J et al (2011) Factors affecting lipid accumulation by Nannochloropsis oculata in a two-stage cultivation process. J Appl Phycol 23:903–908
Sukenik A, Bennett J, Falkowski P (1987) Light-saturated photosynthesis—limitation by electron transport or carbon fixation? Biochim Biophys Acta Bioenerg 891:205–215
Takeshita T, Ota S, Yamazaki T et al (2014) Starch and lipid accumulation in eight strains of six Chlorella species under comparatively high light intensity and aeration culture conditions. Bioresour Technol 158:127–134
Tredici MR (2010) Photobiology of microalgae mass cultures: understanding the tools for the next green revolution. Biogeosciences 1:143–162
Vonshak A, Torzillo G, Tomaseli L (1994) Use of chlorophyll fluorescence to estimate the effect of photoinhibition in outdoor cultures of Spirulina platensis. J Appl Phycol 6:31–34
Walker DA (2009) Biofuels, facts, fantasy, and feasibility. J Appl Phycol 21:509–517
Wellburn AR (1994) The spectral determination of chlorophyll a and chlorophyll b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Williams PJLB, Laurens LML (2010) Microalgae as biodiesel & biomass feedstocks: review & analysis of the biochemistry, energetics & economics. Energy Environ Sci 3:554
Yeh KL, Chang JS (2012) Effects of cultivation conditions and media composition on cell growth and lipid productivity of indigenous microalga Chlorella vulgaris ESP-31. Bioresour Technol 105:120–127
Young E, Beardall J (2003) Photosynthetic function in Dunaliella tertiolecta (Chlorophyta) during a nitrogen starvation and recovery cycle. J Phycol 905:897–905
Yusuf C (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Zhou X, Ge H, Xia L et al (2013) Evaluation of oil-producing algae as potential biodiesel feedstock. Bioresour Technol 134:24–29
Acknowledgments
The authors thank Mr Pavel Souček, Ms Soňa Pekařová and Mr Petr Novotný for technical assistance. The work was supported by the Ministry of Education, Youth and Sports of the Czech Republic, projects AlgaTech CZ.1.05/2.1.00/03.0110, AlgaIn CZ.1.07/2.3.00/30.0059 and AlgaMan CZ.1.07/2.3.00/20.0203. CGJ was financed by the Short-Stage Program of the FPU grant from the Ministry of Education of the Spanish Government. The work was also supported by the research group ‘Photobiology and biotechnology of aquatic organisms’ (RNM-295) financed by Andalusian Government (Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jerez, C.G., Malapascua, J.R., Sergejevová, M. et al. Effect of Nutrient Starvation under High Irradiance on Lipid and Starch Accumulation in Chlorella fusca (Chlorophyta). Mar Biotechnol 18, 24–36 (2016). https://doi.org/10.1007/s10126-015-9664-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-015-9664-6