Abstract
Although the importance of anthozoan-dinoflagellate (genus Symbiodinium) endosymbioses in the establishment of coral reef ecosystems is evident, little is known about the molecular regulation of photosynthesis in the intra-gastrodermal symbiont communities, particularly with respect to the rate-limiting Calvin cycle enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco). In this study, we analyzed rubisco mRNA (rbcL) and protein (RBCL) concentrations over the diel cycle in both cultured and endosymbiotic Symbiodinium samples. In the former, rbcL expression increased upon illumination and decreased during the dark, a pattern that was upheld under continual dark incubation. A different trend in rbcL expression was observed in endosymbiotic Symbiodinium residing within sea anemone (Aiptasia pulchella) tissues, in which illumination gradually led to decreased rbcL mRNA expression. Unexpectedly, RBCL protein expression did not vary over time within anemone tissues, and in neither cultured nor endosymbiotic samples was a correlation between gene and protein expression documented. It appears, then, that photoperiod, lifestyle, and posttranscriptional regulation are all important drivers of RBCL expression in this ecologically important dinoflagellate.



Similar content being viewed by others
References
Badger MR, Andrews TJ, Whitney SM, Ludwig M, Yellowlees DC, Leggat W, Price GD (1998) The diversity and co-evolution of Rubisco, plastids, pyrenoids and chloroplast-based CCMs in the algae. Can J Bot 76:1052–1071
Bellantuono AJ, Hoegh-Guldberg O, Rodriguez-Lanetty M (2011) Resistance to thermal stress in corals without changes in symbiont composition. Proc R Soc Biol Sci Ser B 279:1100–1107
Bruyant F, Babin M, Genty B, Prasil O, Behrenfeld MJ, Claustre H, Bricaud A, Garczareck L, Holtzendorf J, Koblizek M, Dousova H, Partensky F (2005) Diel variations in the photosynthetic parameters of Prochlorococcus strain PCC 9511: combined effects of light and cell cycle. Limnol Oceanogr 50:850–863
Corredor JE, Wawrik B, Paul JH, Tran H, Kerkhof L, López JM, Dieppa A, Cárdenas O (2004) Geochemical rate-RNA in integration study: ribulose-1,5-bisphosphate carboxylase/oxygenase gene transcription and photosynthetic capacity of planktonic photoautotrophs. Appl Environ Microbiol 70:5459–5468
Crawley A, Kline DA, Dunn S, Anthony K, Dove S (2010) The effect of ocean acidification on symbiont photorespiration and productivity in Acropora formosa. Glob Chang Biol 16:851–863
Davies PS (1993) Endosymbiosis in marine cnidarians. In: John DM, Hawkins SJ, Price JH (eds) Plant-animal interactions in the marine benthos. Clarendon, Oxford
Dykens JA, Shick JM (1982) Oxygen production by endo-symbiotic algae controls superoxide dismutase activity in their animal host. Nature 297:579–580
Fukuda I, Imagawa S, Iwao K, Horiguchi T, Watanabe T (2002) Isolation of actin-encoding cDNAs from symbiotic corals. DNA Res 9:217–223
Furla P, Galgani I, Durand I, Allemand D (2000) Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. J Exp Biol 203:3445–3457
Goiran C, Allemand D, Galgani I (1997) Transient Na + stress in symbiotic dinoflagellates after isolation from coral host cells and subsequent immersion in seawater. Mar Biol 129:581–589
Hobson LA, Morris WJ, Guest KP (1985) Varying photoperiod, ribulose 1,5-bisphosphate carboxylase/oxygenase and CO2 uptake in Thalassiosira fluviatilis (Bacillariophyceae). Plant Physiol 79:833–837
Hollnagel HC, Pinto E, Morse D, Colepicolo P (2010) The oscillation of photosynthetic capacity in Lingulodinium polyedrum is not related to differences in RuBisCo, peridinin or chlorophyll a amounts. Biol Rhythm Res 33:443–458
Iglesias-Prieto R, Trench RK (1994) Acclimation and adaptation to irradiance in symbiotic dinoflagellates. I. Responses of the photosynthetic unit to changes in photon flux density. Mar Ecol Prog Ser 113:163–175
Jones RJ, Hoegh-Guldberg O (2001) Diurnal changes in the photochemical efficiency of the symbiotic dinoflagellates (Dinophyceae) of corals: photoprotection, photoinactivation and the relationship to coral bleaching. Plant Cell Environ 24:89–99
Jordan DB, Ogren WL (1981) Species variation in the specificity of ribulose bisphosphate carboxylase/oxygenase. Nature 291:13–15
Kreps JA, Kay SA (1997) Coordination of plant metabolism and development by the circadian clock. Plant Cell 9:1235–1244
Kühl M, Cohen Y, Dalsgaard T, Jorgensen BB, Revsbech NP (1995) Microenvironment and photosynthesis of zooxanthellae in scleractinian corals studies with microsensors for O2, pH and light. Mar Ecol Prog Ser 117:159–172
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
LaJeunesse TC (2002) Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar Biol 141:387–400
LaJeunesse TC, Smith R, Walther M, Pinzon J, Pettay DT, McGinley M, Aschaffenburg M, Medina-Rosas P, Cupul-Magana AL, Perez AL, Reyes-Bonilla H, Warner ME (2010) Host-symbiont recombination versus natural selection in the response of coral-dinoflagellate symbioses to environmental disturbance. Proc R Soc Biol Sci Ser B 277:2925–2934
Leggat W, Badger MR, Yellowlees D (1999) Evidence for an inorganic carbon-concentrating mechanism in the symbiotic dinoflagellate Symbiodinium sp. Plant Physiol 121:247–255
Leggat W, Whitney SM, Yellowlees D (2004) Is coral bleaching due to the instability of the zooxanthellae dark reactions? Symbiosis 37:137–153
Levy O, Achituv Y, Schnider K, Dubinsky Z, Gorbunov M (2004) Diurnal hysteresis in coral photosynthesis. Mar Ecol Prog Ser 268:105–117
Levy O, Achituv Y, Yacobi YZ, Dubinsky Z, Stambler N (2006) Diel “tuning” of coral metabolism: physiological responses to light cues. J Exp Biol 209:273–283
Levy O, Kaniewska P, Alon S, Eisenberg E, Karako-Lampert S, Bay LK, Reef R, Rodriguez-Lanetty M, Miller DJ, Hoegh-Guldberg O (2011) Complex diel cycles of gene expression in coral-algal symbiosis. Science 331:175
Lilley RMC, Ralph PJ, Larkum AWD (2010) The determination of activity of the enzyme Rubisco in cell extractions of the dinoflagellate alga Symbiodinium sp. by manganese chemiluminescence and its response to short-term thermal stress of the alga. Plant Cell Environ 33:995–1004
Mayfield AB, Gates RD (2007) Osmoregulation in anthozoan-dinoflagellate symbiosis. Comp Biochem Physiol A 147:1–10
Mayfield SP, Yohn CB, Cohen A, Danon A (1995) Regulation of chloroplast gene expression. Annu Rev Plant Physiol Plant Mol Biol 46:147–166
Mayfield AB, Hirst MB, Gates RD (2009) Gene expression normalization in a dual-compartment system: a quantitative real-time PCR protocol for symbiotic anthozoans. Mol Ecol Res 9:462–470
Mayfield AB, Hsiao YY, Fan TY, Chen CS, Gates RD (2010) Evaluating the temporal stability of stress-activated protein kinase and cytoskeleton gene expression in the Pacific corals Pocillopora damicornis and Seriatopora hystrix. J Exp Mar Biol Ecol 395:215–222
Mayfield AB, Wang LH, Tang PC, Hsiao YY, Fan TY, et al. (2011) Assessing the impacts of experimentally elevated temperature on the biological composition and molecular chaperone gene expression of a reef coral. PLoS One e26529
Mayfield AB, Chan PH, Putnam HP, Chen CS, Fan TY (2012) The effects of a variable temperature regime on the physiology of the reef-building coral Seriatopora hystrix: results from a laboratory-based reciprocal transplant. J Exp Biol 215:4183–4195
McClung CR (2001) Circadian rhythms in plants. Annu Rev Plant Physiol Plant Mol Biol 52:139–162
Mittag M, Li L, Hastings JW (1998) The mRNA level of circadian regulated Gonyaulax luciferase remains constant over the cycle. Chronobiol Int 15:93–98
Morse D, Milos PM, Roux E, Hastings JW (1989) Circadian regulation of bioluminescence in Gonyaulax involves translational control. Proc Natl Acad Sci U S A 86:172–176
Morse D, Salois P, Markovic P, Hastings JW (1995) A nuclear encoded form II Rubisco in dinoflagellates. Science 268:1622–1624
Moya A, Tambutte S, Beranger G, Gaume B, Scimeca J, Allemand D, Zoccola D (2008) Cloning and use of a coral 36B4 gene to study the differential expression of coral genes between light and dark conditions. Mar Biotechnol 10:653–663
Muscatine L (1990) The role of symbiotic algae in carbon and energy flux in reef corals. In: Dubinsky Z (ed) Ecosystems of the world. Elsevier, Amsterdam
Muscatine L, Grossman D, Doino J (1991) Release of symbiotic algae by tropical sea anemones and corals after cold shock. Mar Ecol Prog Ser 77:233–243
Nassoury N, Fritz L, Morse D (2001) Circadian changes in ribulose-1,5-bisphosphate carboxylase/oxygenase distribution inside individual chloroplasts can account for the rhythm in dinoflagellate carbon fixation. Plant Cell 13:923–934
Paul JH (1996) Carbon cycling: molecular regulation of photosynthetic carbon fixation. Microb Ecol 32:231–245
Paul JH, Pichard SL (1998) Phytoplankton activity through the measurement of ribulose bisphosphate carboxylase gene expression (Rubisco). In: Cooksey KE (ed) Molecular approaches to the study of the ocean. Chapman and Hall, London
Pichard SL, Paul JH (1991) Detection of gene expression in genetically engineered microorganisms and natural phytoplankton populations in the marine environment by mRNA analysis. Appl Environ Microbiol 51:1721–1727
Pichard SL, Paul JH (1993) Gene expression per gene dose: a specific measure of gene expression in aquatic microorganisms. Appl Environ Microbiol 59:451–457
Pichard SL, Campbell L, Kang JB, Tabita FR, Paul JH (1996) Regulation of ribulose bisphosphate carboxylase expression in natural phytoplankton communities. I. Diel rhythms. Mar Ecol Prog Ser 139:257–265
Pichard SL, Campbell L, Carder K, Kang JB, Patch J, Tabita FR, Paul JH (1997) Analysis of ribulose bisphosphate carboxylase gene expression in natural phytoplankton communities by group-specific gene probing. Mar Ecol Prog Ser 149:239–253
Pochon X, Putnam HM, Burki F, Gates RD (2011) Identifying and characterizing alternative molecular markers for the symbiotic and free-living dinoflagellate genus Symbiodinium. PLoS ONE e29816
Porter JW, Muscatine L, Dubinsky Z, Falkowski PG (1984) Primary production and photoadaptation in light-adapted and shade-adapted colonies of the symbiotic coral, Stylophora pistillata. Proc R Soc Biol Sci Ser B 222:161–180
Rowan R, Powers DA (1991) Molecular genetic identification of symbiotic dinoflagellates (zooxanthellae). Mar Ecol Prog Ser 71:65–73
Seibt C, Schlichter D (2001) Compatible intracellular ion composition of the host improves carbon assimilation by zooxanthellae in mutualistic symbioses. Naturwissenschaften 88:382–386
Sorek M, Levy O (2012) Influence of the quantity and quality of light on photosynthetic periodicity in coral endosymbiotic algae. PLoS ONE e43264
Stat M, Pochon X, Cowie ROM, Gates RD (2009) Specificity in communities of Symbiodinium in corals from Johnston Atoll. Mar Ecol Prog Ser 386:83–96
Stat M, Bird CE, Pochon X, Chasqui L, Chauka LJ, Concepcion GT, Logan D, Takabayashi M, Toonen RJ, Gates RD (2010) Variation in Symbiodinium ITS2 sequence assemblages among coral colonies. PLoS ONE e15854
Tolosa JM, Schjenken JE, Civiti TD, Clifton VL, Smith R (2007) Column-based method to simultaneously extract DNA, RNA, and proteins from the same sample. Biotechniques 43:799–804
van Dolah FM, Lidie K, Morey J, Brunelle S, Ryan J, Monroe E, Haynes B (2007) Microarray analysis of diurnal and circadian regulated genes in the Florida red tide dinoflagellate, Karenia brevis. J Phycol 43:741–752
Wang LH, Liu YH, Ju YM, Hsiao YY, Fang LS, Chen CS (2008) Cell cycle propagation is driven by light-dark stimulation in a cultured symbiotic dinoflagellate isolated from corals. Coral Reefs 27:823–835
Whitney SM, Andrews TJ (1998) The CO2/O2 specificity of single-subunit ribulose-bisphosphate carboxylase from the dinoflagellate, Amphidinium carterae. Aust J Plant Physiol 25:131–138
Whitney SM, Yellowlees D (1995) Preliminary investigations into the structure and activity of ribulose bisphosphate carboxylase from two photosynthetic dinoflagellates. J Phycol 31:138–146
Whitney SM, Shaw DC, Yellowlees D (1995) Evidence that some dinoflagellates contain a ribulose-1,5-bisphosphate carboxylase/oxygenase related to that of the α-proteobactineria. Proc R Soc Biol Sci Ser B 259:271–275
Wyman M, Davies JT, Weston K, Crawford DW, Purdie DA (1998) Ribulose-1,5-carboxylase/oxygenase (Rubisco) gene expression and photosynthetic activity in nutrient-enriched mesocosm experiments. Estuar Coast Shelf Sci 46:23–33
Xu HH, Tabita FR (1996) Ribulose-1,5-bisphosphate carboxylase/oxygenase gene expression and diversity of Lake Erie planktonic microorganisms. Appl Environ Microbiol 62:1913–1921
Yang YW (2001) Polymorphic symbiosis and phylogenetic analysis of zooxanthellae in the Indo-Pacific scleractinian corals. Master’s thesis. National Sun Yat-Sen University, Kaohsiung, Taiwan
Zhang H, Lin S (2003) Complex gene structure of the form II Rubisco in the dinoflagellate Prorocentrum minimum (Dinophyceae). J Phycol 39:1160–1171
Zhang H, Hou Y, Miranda L, Campbell DA, Sturm NR, Gaasterland T, Lin S (2007) Spliced leader RNA trans-splicing in dinoflagellates. Proc Natl Acad Sci U S A 104:4618–4623
Zinser ER, Lindell D, Johnson ZI, Futschik ME, Steglich C, Coleman ML, Wright MA, Rector T, Steen R, McNulty N, Thompson LR, Chisholm SW (2009) Choreography of the transcriptome, photophysiology, and cell cycle of a minimal photoautotroph, Prochlorococcus. PLoS ONE e5135
Acknowledgments
ABM was funded by an international research fellowship from the United States National Science Foundation (OCE-0852960) and the Khaled bin Sultan Living Oceans Foundation. NMMBA and National Science Council (NSC 98-2311-B-291-001-MY3 and 101-2311-B-291-002-MY3) provided funds to CSC that were instrumental to the success of the laboratory analyses.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.

Fig. s1
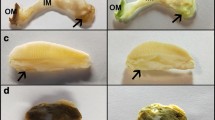
Primer specificity validation study. (A) Symbiodinium density in Aiptasia pulchella specimens of different symbiotic status (healthy, partially bleached, and fully bleached) was observed with DIC microscopy. (B) The same samples as in (A) were also imaged under a fluorescent microscope. The red fluorescence is from the chlorophyll of the Symbiodinium populations while the green represents autofluorescence of the host. (C) Selected insets in (B) were magnified to better demonstrate Symbiodinium density in hospite. (D) Neither rbcL nor act1 mRNAs were detected in partially and fully bleached specimens after 27 PCR cycles. In partially bleached A. pulchella, only the act1 mRNA could be detected after 40 cycles. (JPEG 191 kb)
Fig. s2
RFLPs. A portion of the Symbiodinium 18s rDNA gene was amplified with PCR, digested with either TaqI or Sau3 AI, and electrophoresed as described in the text. Two clade B Symbiodinium samples from a previous study (Wang et al. 2008) are shown as a reference (A), and four representative samples are shown for each of the following groups: the Symbiodinium cultures (B), anemones of the 12L:12D study (C), and anemones of the primer specificity validation study (PSVS; D). In all panels, lanes 1 and 4, and, when applicable, 7 and 10, represent the undigested, ~1,500 bp PCR product. Lanes 2 and 5, and, when applicable, 8 and 11, represent TaqI-digested amplicons. Lanes 3 and 6, and, when applicable, 9 and 12, represent Sau3 AI-digested amplicons. DNA ladders are shown in each panel. (AI 1025 kb)
Appendixes
Appendixes
Appendix 1 Primer Specificity Validation Study
Materials and Methods
Endosymbiotic sea anemones with an oral disc size of ~0.5 cm were collected and grown in 250 ml glass flasks containing 200 ml sterile filtered seawater (SFSW) for several months, and one specimen from each of three randomly selected clonal cultures was partially dissected to remove one tentacle for DNA extraction (described in Appendix 2), which was snap-frozen in a 1.5-ml microcentrifuge tube and stored at −80 °C. The partially dissected anemones were then visualized under a fluorescent microscope, snap-frozen in liquid nitrogen, and stored at −80 °C prior to extraction of their RNAs (described in the main article text) to serve as the healthy group (n = 3). Images were obtained with a DAPI filter set (UV/Violet; Ex: BP 365/12 nm; FT 395 nm; LP 397 nm) on a Zeiss dissecting microscope (SteREO Discovery V8, Carl Zeiss International, Germany) using a Zeiss Axiocam digital camera to detect Symbiodinium (red) chlorophyll fluorescence.
Six randomly selected anemone cultures were incubated at 4 °C for 2 h to induce bleaching (sensu Muscatine et al. 1991). After cold shock, one partially bleached anemone from each of the three randomly chosen cold-shocked cultures was dissected and visualized under the fluorescent microscope as described above, snap-frozen in liquid nitrogen, and stored at −80 °C to serve as the partially bleached group (n = 3). The remaining cold-shocked specimens were transferred to new flasks containing SFSW and then kept at 25–28 °C in total darkness for 8 months. The SFSW was replaced twice weekly, and, once Symbiodinium were no longer visible under the fluorescent microscope, one anemone from each of the three fully bleached clonal populations was imaged, dissected as above, snap-frozen in liquid nitrogen, and kept at −80 °C prior to extraction of RNAs to serve as the fully bleached group (n = 3). RNA extraction, cDNA synthesis, and SQ-RT-PCRs were conducted with the nine samples as described in the main article text.
Results
Symbiodinium density was diminished in partially bleached anemones, and in fully bleached specimens, no Symbiodinium were microscopically detected (Fig. s1a–c, right-most panels). act1 and rbcL cDNAs were amplified by PCR for either 27 or 40 cycles (Fig. s1d). As fewer Symbiodinium were found in partially bleached samples, decreased expression of rbcL and act1 was evident; PCR for 27 cycles did not amplify either gene at a detectable level (Fig. s1d). Detection of act1 expression in partially bleached A. pulchella specimens could only be achieved after 40 PCR cycles. Furthermore, neither rbcL nor act1 transcripts were detected in fully bleached anemones, even after 40 PCR cycles (Fig. s1d).
Appendix 2 RFLP Analysis of Cultured and Endosymbiotic Symbiodinium
To determine the genetic identity to clade level for both cultured and endosymbiotic Symbiodinium used in the study, DNAs were extracted from ~106 cells from each of the 12 Symbiodinium cultures from which the experimental aliquots were removed, the individual tentacles dissected from each of the 9 anemones sampled for the primer specificity validation study (PSVS, Appendix 1), and the 24 A. pulchella (entire anemone) specimens sampled at 3-h intervals across one 12 L:12D cycle with the AxyPrep™ Multisource Genomic DNA Miniprep kit (Axygen Biosciences, CA, USA) according to the manufacturer’s recommendations after homogenizing the cells, tentacles, and anemones, respectively, in liquid nitrogen with a mortar and pestle. The 45 DNAs were PCR-amplified with the Symbiodinium 18 s rDNA primers (“ss3z-ss5z”), reagent concentrations, and thermocycling conditions of Rowan and Powers (1991), and the PCR products were excised from the gel with a razor blade and purified with the AxyPrep™ DNA gel extraction kit (Axygen Biosciences) according to the manufacturer’s recommendations.
The purified PCR amplicons were then digested with TaqI and Sau3 AI (New England Biolabs, MA, USA) in separate 20 μl reactions as in Yang (2001), and 10 μl of the digestion products were electrophoresed on 1.5 % tris-acetate-EDTA (TAE) agarose gels, stained in an ethidium bromide (EtBr) bath for 20 min, visualized at 610 nm on a Typhoon Trio™ Scanner (GE Healthcare, WI, USA), and the digestion patterns compared to those of Rowan and Powers (1991) and Yang (2001) to verify Symbiodinium identity in each of the 45 DNA samples. Subcladal diversity of Symbiodinium (sensu LaJeunesse 2002; LaJeunesse et al. 2010; Bellantuono et al. 2011) was not assessed, as the markers used to infer such diversity, particularly its2, are intragenomically variable (Stat et al. 2009, 2010) and hence unable to resolve subcladal genetic differences (Pochon et al. 2011).
Rights and permissions
About this article
Cite this article
Mayfield, A.B., Hsiao, YY., Chen, HK. et al. Rubisco Expression in the Dinoflagellate Symbiodinium sp. Is Influenced by Both Photoperiod and Endosymbiotic Lifestyle. Mar Biotechnol 16, 371–384 (2014). https://doi.org/10.1007/s10126-014-9558-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10126-014-9558-z