Abstract
Background
Diffuse-type gastric cancer (DGC), for which Helicobacter pylori infection is a causal factor, is associated with poor prognosis among young women, possibly due to female hormones such as estrogen. We aimed to identify the carcinogenesis induced by estrogen and H. pylori in DGC.
Methods
We screened and selected estrogen receptor alpha (ERα)-positive (MKN45) and ERα-negative (SNU5) DGC cell lines. H. pylori strain 60190 and its isogenic mutant strain lacking cytotoxin-associated gene A (60190ΔCagA) were used to infect MKN45 cells. And the cytotoxin-related gene A (CagA) cDNA which was cloned into pSP65-SR-HA (cagA-pSP65SRa) vector was used to transfect MKN45 cells. Tumor samples were used for DGC organoid culture.
Results
In MKN45 cells, we found that estradiol promotes epithelial–mesenchymal transition (EMT) and stemness phenotypes via HOTAIR expression. These effects were further enhanced by the addition of CagA secreted by H. pylori but were reversed by co-treatment with fulvestrant (ICI 182,780), a selective ER degrader. We also validated the effect of estrogen on DGC organoids. ERα expression was associated with tumor invasion and HOTAIR expression in DGC patients with overt H. pylori infection.
Conclusions
These findings may explain the rapid DGC progression in young women with physiologically high levels of estrogen and suggest that fulvestrant with ovarian function suppression could serve as a tumor-suppressive agent in premenopausal patients with DGC.
Similar content being viewed by others
Introduction
Gastric cancer is a globally impactful cancer, with 1,000,000 new cases and 783,000 deaths in 2018, making it the fifth most common diagnosed cancer and third leading cause of cancer-related death [1, 2]. One-twelfth of cancer-related deaths worldwide are attributed to stomach cancer [1]. Until the mid-1990s, stomach cancer was the most common cause of cancer-related death, but over the past 50 years, reduced Helicobacter pylori infections and changes in food preservation may have decreased the overall incidence of gastric cancer [3]. This decrease in incidence, however, has been mainly limited to intestinal-type gastric cancer (IGC), whereas the incidence of diffuse-type gastric cancer (DGC) has remained constant [4, 5]. Compared with IGC, DGC more frequently affects young female patients [6, 7] and is often found at more advanced stages [8]. Moreover, these young female DGC patients have a higher mortality rate [9]. One reason for the high incidence of DGC in young women may be its association with female hormones such as estrogen. Although several studies have examined the expression of sex hormone receptors and their prognostic implications in gastric cancer, their results remain inconclusive and controversial [5, 10,11,12,13,14]
Previous reports indicate that H. pylori infection is closely related to IGC, but it is also believed to be a causal factor for DGC [15, 16]. DGC is more strongly associated with overt or relatively recent H. pylori infection rather than past infection [17]. In addition, a recent study of 917 patients with gastric cancer reports that the titer of antibody to H. pylori was higher in DGC than in IGC [8], suggesting that H. pylori might be more closely related to DGC than to IGC [8, 18, 19]. Therefore, we aimed to identify the tumorigenic mechanisms of DGC induced by estrogen and H. pylori.
Materials and methods
Human tissue samples and clinical data
Gastric cancer tissues from 57 adult patients undergoing gastrectomy due to advanced DGC adenocarcinoma between March 2010 and December 2015 were provided by the tumor bank of the National Cancer Center of Korea and retrospectively analyzed. These patients were treated with open or laparoscopy-assisted gastrectomy with D2 lymph node (LN) dissection. The extent of LN dissection followed recommendations of the Korean Practice Guidelines for Gastric Cancer [20]. Cancer stage after surgery was evaluated according to the 8th edition of the American Joint Committee on Cancer TNM staging system [21]. Clinicopathologic characteristics including age, sex, H. pylori infection status, tumor characteristics, TNM stage, and death were obtained by review of medical charts. H. pylori infection status was confirmed using a rapid urease test, histopathologic examination, and detection of H. pylori IgG antibody. Positive results from either the rapid urease test or histopathologic examination were considered to indicate current infection with H. pylori. If only H. pylori IgG antibody was positive, this was considered to indicate a past infection. Informed written consent was obtained from all patients, and the study was exempted from review by the Institutional Review Board of the National Cancer Center of Korea because it involved minimal risk due to the use of existing specimens (NCCTTR-17007).
DGC cell line culture and reagents
The DGC cell lines SNU1, SNU5, SNU16, SNU484, SNU601, SNU638, and MKN45 and the IGC cell lines AGS, NCI-N87, MKN1, MKN74, SNU216, and SNU719 were obtained from the Korean Cell Line Bank (Seoul, Korea). Normal gastric epithelial cell line HFE145 was provided by Drs. Hassan Ashktorab and Duane T. Smoot (Howard University, Washington, DC). HFE145 epithelial cells displayed female-specific chromosome content [22] and were estrogen receptor positive.
For estradiol treatment, cells were maintained for at least three passages in phenol red-free RPMI-1640 supplemented with 10% dextran-coated charcoal-treated FBS, penicillin, and streptomycin in a humidified incubator with 5% CO2 at 37 °C. Cells were grown to 70% confluency, treated with 17-β-estradiol (E8875, Sigma-Aldrich, St. Louis, MO, USA) and ICI 182,780 (Fulvestrant; Sigma-Aldrich), and incubated for 3, 6, or 24 h as previously described [23].
RNA extraction and qPCR
For qPCR analysis, RNA was extracted from cells using an RNA prep kit (PureLink™ RNA Mini Kit; Thermo Fisher Scientific, Waltham, MA, USA), and cDNA was synthesized using a PrimeScript™ 1st Strand cDNA Synthesis Kit (Takara Bio Inc., Kusatsu, Japan). cDNA was amplified using Ampigene qPCR Green Mix Hi-ROX with a StepOne™ Real-Time PCR System (Thermo Fisher Scientific). The sequencing primers are listed in Supplementary Table S1.
Western blotting
Cells were prepared as previously described [24]. Primary antibodies for ERα (sc-514857), CagA (sc-28368), and GAPDH (sc-47724) (all Santa Cruz Biotechnology, Santa Cruz, CA, USA); Zo1 (#8193), E-cad (#3195), N-cad (#4061), Snail (#3879), Slug (#9585), Vimentin (#5741), Oct4 (#2750), and Nanog (#4893) (all Cell Signaling, Danvers, MA, USA); and C-myc (A190-105A) (Bethyl, Montgomery, TX, USA) were used for protein detection as previously described [25].
Viability assay
After MKN45 cells were seeded and incubated overnight, they were treated with estradiol or ICI 182,780. Cell viability was measured for 2 days using WST-1 ((4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate; Takara Bio Inc.) according to the manufacturer’s protocol.
H. pylori infection and CagA vector
H. pylori (60190) was provided by Prof. Yong Chan Lee of Yonsei University, Korea. Isogenic mutants lacking CagA (60190ΔCagA) were used as described previously [26, 27]. MKN45 and SNU638 cells were cultured for 24 h and infected with H. pylori (60190) or 60190ΔCagA at a multiplicity of infection of 100:1 for 4 or 24 h. The full-length CagA gene of H. pylori standard strain NCTC1167, which was cloned into a pSP65SRa vector, was also provided by Prof. Yong Chan Lee. For plasmid transfection, MKN45 cells were seeded until 70% confluency and transfected with pSR665SRa-CagA vector for 24 h using LT1 (MIR 2304, Takara Bio Inc.).
In vitro migration and invasion assay
Migration and invasion assay was performed with estradiol- or ICI 182,780-treated MKN45 cells and diffuse-type gastric cancer organoids using 8.0-µm pore inserts in a 24-well Transwell apparatus (Corning, Corning, NY, USA) following the manufacturer’s instructions and as previously described [23].
Immunofluorescence
Primary antibodies were mouse anti-Ki67 (sc-101861, Santa Cruz Biotechnology), rabbit anti-E-cad (#3195, Cell Signaling), and rabbit anti-KLF5 (Biorbyt, orb107539, Cambridgeshire, UK). Secondary antibodies were rabbit-FITC (A120-101F, Bethyl) and mouse-Dylight 550 (A120-101D3, Bethyl). Immunofluorescence was performed as previously described [27].
Sphere formation assay
MKN45 cells were plated onto an ultra-low attachment 6-well plate (Corning) at 1000 cells per well. Cells were cultured in cancer stem cell medium (Supplementary Table S2) for 14 days. Cultured spheres (> 100 µm) were treated with estradiol or ICI 182,780.
Luciferase reporter assays
Luciferase assays were performed with harvested cell extracts using a Dual luciferase assay kit (Promega, Madison, WI, USA). The relative activity of firefly luciferase was normalized by Renilla luciferase activity as previously described [27]. To confirm estrogen-induced HOTAIR promoter activity, four EREs in a 2000-bp fragment of the HOTAIR promoter were amplified using PCR and cloned into a pGL3.basic luciferase vector (Promega). The sequencing primers are listed in Supplementary Table S3.
HOTAIR overexpression vector
HOTAIR cDNA was inserted into a LZRS vector to create a HOTAIR overexpression vector (#26110, Addgene, Watertown, MA, USA). MKN45 and HFE145 cells were grown to 70% confluence and transfected with HOTAIR-cDNA vector using LT1 reagent (MIR 2304, Takara Bio Inc.).
siRNA transfection
MKN45 cells were cultured in serum-free medium at 37℃ until to grow to 70% confluence. Next day, the cells were transfected with negative control siRNA (SN-1012) and si-HOTAIR using TransIT-X2 Dynamic Delivery System (Mirus). After 48 h, cells treated with 17-β-estradiol, and incubated for 3 h. The siRNA sequences for HOTAIR are listed in Supplementary Table S4.
Organoid model
Organoids were constructed from tumor tissue obtained from two DGC patients: one with ERα-positive and the other with ERα-negative organoids. This procedure was approved by the Institutional Review Board of Seoul National University Hospital (IRB #1901-166-1007 & #H-2203-009-1303). Approximately 5 mm3 tissue was cut into small pieces of ~ 1 mm and washed with PBS. For digestion, pieces were incubated for 1 h at 37 °C with chelating solution (Supplementary Table S2). After centrifuging at 500 g for 5 min, the supernatant was removed, and tissue pieces were resuspended in Matrigel (534230, Sigma-Aldrich). For complete polymerization of Matrigel, the plate was placed in a CO2 incubator at 37 °C for 15 min, and complete organoid culture medium (Supplementary Table S2) were added over the Matrigel.
Statistics
Statistical Package for the Social Sciences version 25 (IBM SPSS Inc, Chicago, IL, USA) was used for statistical analysis. All experiments were performed in triplicate, and results are presented as mean ± standard deviation. Mann–Whitney U tests were used to detect significant differences between experimental and control groups. Pearson correlation coefficients were used to compare HOTAIR expression patterns between tumor and normal tissue. A P value < 0.05 was considered statistically significant.
Results
Estrogen induces epithelial–mesenchymal transition and a stemness phenotype in DGC cells
We first evaluated the expression of estrogen receptor α (ERα) in various DGC cell lines (i.e., SNU1, SNU5, SNU16, SNU484, SNU638, MKN45, and SNU601). ERα was more highly expressed in four DGC cell lines (i.e., SNU1, SNU16, SNU638, and MKN45) than in three other DGC cell lines (i.e., SNU5 SNU484, and SNU601). Therefore, we selected MKN45 and SNU638 as ERα-positive DGC cell lines and SNU5 as an ERα-negative DGC cell line for further analysis (Fig. 1a). Next, we also evaluated the expression of estrogen receptor α (ERα) in various IGC cell lines (i.e., AGS, NCI-N87, MKN1, MKN 74, SNU216, and SNU719). ERα was more highly expressed in four IGC cell lines (i.e., NCI-N87, MKN74, SNU216, and SNU719) than in two other IGC cell lines (i.e., AGS and MKN1). We selected SNU216 as an ERα-positive DGC cell line and AGS as an ERα-negative IGC cell line for further analysis (Fig. 1b). Treatment with 0.1 nM estradiol did not alter the viability of SNU5 cells (Fig. 1c) but increased the viability of MKN45 and SNU638 cells (Fig. 1d, e). To check whether this increase in cell viability was caused by estradiol, cells were co-treated with estradiol and 10 μM ICI 182,780 (fulvestrant), a selective ER degrader. This co-treatment reversed MKN45 and SNU638 cell viability, indicating that estradiol was responsible for increasing cell viability (P = 0.0021 (control vs. E2), P = 0.7929 (control vs. E2 + ICI 182,780), and P < 0.001(E2 vs. E2 + ICI 182,780), Fig. 1d; P < 0.001 (control vs. E2), P = 0.7994 (control vs. E2 + ICI 182,780), and P = 0.0014(E2 vs. E2 + ICI 182,780), Fig. 1e). Treatment with 0.1 nM estradiol did not alter the viability of IGC cells such as AGS (Fig. 1f) and SNU216 (Fig. 1g). In addition, expression of the cell proliferation marker Ki67 was increased in estradiol-treated MKN45 cells but not in estradiol and ICI 182,780 co-treated cells (P = 0.0057 (control vs. E2), P = 0.5630 (control vs. E2 + ICI 182,780), and P < 0.001 (E2 vs. E2 + ICI 182,780), Fig. 1h).
Estrogen increases cell viability, migration, and invasion and induces EMT and a stem cell phenotype in DGC cells. a Baseline expression of ERα in seven DGC cell lines. MKN45 and SNU638 were selected as an ERα-positive DGC cell line and SNU5 was selected as an ERα-negative DGC cell line. b Baseline expression of ERα in six IGC cell lines. SNU216 was selected as an ERα-positive IGC cell line and AGS was selected as an ERα-negative IGC cell line. c Estradiol did not affect the cell viability of SNU5 cells. d, e Estradiol increased the cell viability of MKN45 cells and SNU638 cells by more than twofold, which was reversed by co-treatment with ICI 182,780. f, g Estradiol did not affect the cell viability of IGC cells such as AGS and SNU216. h Estradiol increased Ki67 expression in MKN45 cells by twofold, which was reversed by co-treatment with ICI 182,780. i, j Estradiol increased migration and invasion rate of MKN45 and SNU638 cells, which was reversed by co-treatment with ICI 182,780. k, l Estradiol did not affect the migration and invasion of IGC cells, AGS and SNU216. m When expression of EMT markers was examined using western blot, estradiol-treated MKN45 cells showed decreased E-cadherin and increased N-cadherin, Snail, and Vimentin. n Estradiol-treated SNU638 cells showed decreased E-cadherin and increased Snail, Slug. o Immunofluorescence assay shows that estradiol decreased E-cadherin, which was reversed by co-treatment with ICI 182,780. p Estradiol-treated MKN45 cells showed increased expression of the stemness markers, C-Myc, Nanog, FST, and SALL4. q Estradiol-treated SNU638 cells showed increased C-Myc and Oct4. r, s Estradiol increased expression of the gastric cancer stem cell markers SOX2 and KLF5. t MKN45 cell spheroids showed increased expression of stemness markers CD44 and Sox2, Oct4 after estradiol treatment, which was reversed by co-treatment with ICI 182,780. u Estradiol increased the size of spheroids and CD44 expression. EMT, epithelial mesenchymal transition; DGC diffuse gastric cancer, ER estrogen receptor, IGC intestinal gastric cancer. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control
We next examined the effect of estradiol on epithelial–mesenchymal transition (EMT) in DGC and IGC cell lines. Migration and invasion rates of ERα-positive DGC cell lines (MKN45, SNU638) increased after estradiol treatment but not after co-treatment with estradiol and ICI 182,780 (P = 0.0021 (control vs. E2), P = 0.7929 (control vs. E2 + ICI 182,780), and P < 0.001 (E2 vs. E2 + ICI 182,780) for migration of MKN45; P = 0.0019 (control vs. E2), P = 0.1270 (control vs. E2 + ICI 182,780), and P = 0.0032 (E2 vs. E2 + ICI 182,780) for invasion of KN45, Fig. 1i; P < 0.001 (control vs. E2), P = 0.9534 (control vs. E2 + ICI 182,780), and P < 0.001 (E2 vs. E2 + ICI 182,780) for migration of SNU638; P < 0.001 (control vs. E2), P = 0.5675 (control vs. E2 + ICI 182,780), and P < 0.001 (E2 vs. E2 + ICI 182,780) for invasion of SNU638, Fig. 1j). However, estradiol did not affect the migration and invasion of IGC cells [AGS (Fig. 1k), SNU216 (Fig. 1l)].When we measured the expression of EMT markers using western blotting, we found that estradiol-treated MKN45 cells showed decreased levels of E-cadherin and increased levels of N-cadherin, Snail, and Vimentin (Fig. 1m). In addition, estradiol-treated SNU638 cells showed decreased levels of E-cadherin and increased levels of Snail and Slug (Fig. 1n). This trend in the expression of EMT markers was not observed in the IGC cell lines (Supplementary Fig. S1a, b). As shown by immunofluorescence assay, the level of E-cadherin decreased only after estradiol treatment alone but not after co-treatment with estradiol and ICI 182,780 (P < 0.001 (control vs. E2), P = 0.3187 (control vs. E2 + ICI 182,780), and P = 0.0032 (E2 vs. E2 + ICI 182,780), Fig. 1o).
To confirm whether a stem cell phenotype was induced by estradiol in ERα-positive DGC cells, we measured the expression of stemness markers using western blotting. Estradiol enhanced the expression of C-Myc, Nanog, FST, and SALL4 in MKN45 cells (Fig. 1p). In addition, Estradiol-treated SNU638 cells showed increased C-Myc and Oct4 (Fig. 1q). In contrast, the expression of stemness markers in the IGC cell lines did not show such a trend (Supplementary Fig. S1c, d). In addition, immunofluorescence assays showed that estradiol-treated MKN45 cells exhibited increased expression of SOX2 (Fig. 1r) and KLF5 (Fig. 1s), which is related to stemness [28].
In western blot analysis of spheroid cultures of MKN45 cells, the expression of CD44, Sox2, and Oct4 increased after estradiol treatment alone but not after co-treatment with estradiol and ICI 182,780 (Fig. 1t). In addition, immunofluorescence assay showed that estradiol increased the size of spheroids (P = 0.0089 (control vs. E2), P = 0.1920 (control vs. E2 + ICI 182,780), and P = 0.0025 (E2 vs. E2 + ICI 182,780), Fig. 1u). Together, these findings suggest that estradiol promotes migration and invasion ability and induces EMT and stemness phenotypes in the presence of ERα in DGC cells.
Estrogen and its coregulators induce HOTAIR transcription in DGC cells
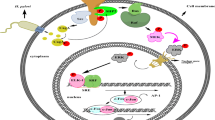
Recent studies show that long non-coding RNAs play an important role in human cancer [29]. Here, we focused on HOX antisense intergenic RNA (HOTAIR) due to its role as an oncogene in several solid tumors such as those of the breast, lung, and liver [30]. In addition, the promoter of HOTAIR contains multiple estrogen response elements (EREs). ERs and their coregulators, including mixed-lineage leukemia protein 3 (MLL3), promote estradiol-induced transcriptional activation of HOTAIR in ERα-positive breast cancer cells [31]. Therefore, we hypothesized that estrogen-induced transcription of HOTAIR in the presence of ERα contributes to the progression of DGC (Fig. 2a).
Estrogen-induced transcription of HOTAIR occurs in the presence of ERα and its coregulators. a We hypothesized that estradiol-induced HOTAIR transcription is modulated by ERα. b, c Estradiol increased expression of an oncogenic lncRNA, HOTAIR, by twofold in MKN45 and SNU638 cells, which was reversed by co-treatment with ICI 182,780. d, e In IGC cell lines, estradiol did not affect the expression of HOTAIR regardless of presence of absence of ERα expression. f Luciferase assay confirmed that the HOTAIR promoter contains four ERE binding sites, with the ERE2 and ERE3 regions being more highly induced by estradiol. g, h HOTAIR was overexpressed in MKN45 and HFE145 cells using the HOTAIR overexpression vector. i, j Overexpression of HOTAIR induced EMT and stemness markers in MKN45 and HFE145 cells. k si-HOTAIR downregulated the expression of HOTAIR in MKN45 cells. When HOTAIR expression was suppressed, E-cad expression increased and Snail expression decreased even after estradiol treatment. HOTAIR HOX antisense intergenic RNA, lncRNA long non-coding RNA, IGC intestinal gastric cancer, ER estrogen receptor, ERE estrogen response element. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control
Real-time polymerase chain reaction (qPCR) analysis showed that the expression of HOTAIR increased by twofold when MKN45 and SNU638 cells were treated with estradiol but not when co-treated with estradiol and ICI 182,780 (P = 0.0082, control vs. E2, P = 0.1452, control vs. E2 + ICI 182,780, Fig. 2b; P = 0.0120, control vs. E2, P = 0.0564, control vs. E2 + ICI 182,780, Fig. 2c). In the case of IGC cell lines, there was no statistically significant difference in the expression of HOTAIR when estradiol was treated regardless of ERα positive or negative (Fig. 2d, e). To determine whether estrogen directly regulates HOTAIR expression via ERα, we examined the promoter of HOTAIR. We identified four binding sites (ERE1, ERE2, ERE3, and ERE4) within the HOTAIR promoter region. Next, we cloned luciferase vectors (ERE-pGL3.B) fused individually with each ERE to allow ERE-pGL3.B or empty-pGL3.B to be co-transfected with Renilla and luciferase vector into MKN45 cells. ERE2 and ERE3 regions showed the highest luciferase activity upon estradiol treatment, suggesting that the HOTAIR promoter contains two functional EREs that are sensitive to estradiol and could potentially contribute to estradiol-mediated transcriptional activation of HOTAIR. These results confirm that HOTAIR expression is estrogen-dependent (Fig. 2f).
To confirm the role of HOTAIR in gastric cancer, HOTAIR was overexpressed in MKN45 cells and the normal human gastric epithelial cell line HFE145. We first used qPCR to confirm that HOTAIR was overexpressed in both cell lines (P = 0.0021 (control vs. E2) in MKN45, Fig. 2g), P < 0.001 (control vs. E2) in HFE145, Fig. 2h). We then used western blotting to examine EMT and stemness markers in HOTAIR-overexpressing MKN45 and HFE145 cells and found decreased levels of E-cadherin and increased levels of Snail and Slug (Fig. 2i, j). These results suggest that the estrogen-induced EMT phenotype of MKN45 cells is mediated by HOTAIR expression. HOTAIR was downregulated with si-HOTAIR to evaluate its effects in MKN45 cell line. When HOTAIR expression was suppressed, the expression of E-cad increased and the expression of Snail was decreased even after treatment with estradiol. As a result, even when MKN45 was treated with estradiol, when HOTAIR expression was knocked down, the EMT change shown in Fig. 1 was not seen (Fig. 2k).
CagA vector and estradiol co-treatment enhances EMT and stem cell phenotypes in DGC cells
H. pylori causes IGC that progresses through the stages of chronic atrophic gastritis and intestinal metaplasia [32]. H. pylori is also closely associated with DGC [16]. In particular, CagA, an oncoprotein secreted by H. pylori, enters gastric epithelial cells through a type IV secretion system and plays a key role in cancer development [27], although the precise underlying mechanisms are unclear. We first infected SNU638 cells with H. pylori strain 60190, and evaluated the mRNA level of well-known coregulators of ER complex, including CBP, p300, MLL1, and MLL3 when SNU 638. MLL1 and MLL3 were highly expressed in cells infected with H. pylori strain 60190. Notably, MLL3, a coregulator of the ER complex, was expressed more highly in cells infected with H. pylori (60190) than cells infected with 60190ΔCagA compared to MLL1 whose change was not observed in cells treated with 60190ΔCagA (Fig. 3a). This suggests that the recruitment of MLL3 is mediated through CagA, an oncoprotein secreted by H. pylori. Therefore, we selected MLL3 among the coregulators of ER complex. Next, MKN45 cells were also infected with H. pylori (61090) or CagA-deficient H. pylori 60190 (60190 ΔCagA). CagA protein was detected only in MKN45 cells infected with H. pylori (60190) and not in cells infected with 60190ΔCagA. Notably, MLL3, a coregulator of the ER complex, was expressed more highly in MKN45 cells infected with H. pylori (60190) than cells infected with 60190ΔCagA, suggesting that expression of MLL3 is induced by H. pylori-secreted CagA (Fig. 3b). When MKN45 cells were infected with H. pylori (60190), EMT markers such as Snail, Slug, and Vimentin were highly expressed (Fig. 3c), and the cell migration rate increased. These effects were not observed when cells were infected with 60190ΔCagA (P < 0.001 (control vs. 60190), P = 0.3739 (control vs. 60190ΔCagA), and P < 0.001 (60190 vs. 60190ΔCagA), Fig. 3d). To test whether H. pylori and estrogen have a synergistic effect, we attempted to simultaneously treat MKN45 cells with H. pylori and estradiol. However, when cells were infected with H. pylori and treated with estradiol simultaneously, they tended to become necrotic. Therefore, we replaced H. pylori infection with CagA-expressing vector transfection. When MKN45 cells were transfected with CagA-expression vector, CagA protein was detected by western blot, demonstrating the effectiveness of replacing H. pylori infection with CagA vector transfection (Fig. 3e).
Expression of EMT and stemness markers increases upon co-treatment with estradiol and CagA vector. a Coregulators of ER complex, including CBP, p300, MLL1, and MLL3 were evaluated in H. pylori-infected SNU638 cells. MLL1 and MLL3 were highly expressed in cells infected with H. pylori strain 60190. MLL3 expression was reduced in cells infected with 60190ΔCagA compared to MLL1 whose change was not observed in cells treated with 60190ΔCagA, which suggests recruitment of MLL3 is mediated through CagA. b CagA-positive H. pylori recruited MLL3, a coregulator of the ER complex. c Expression of EMT markers such as Snail, Slug, and Vimentin increased after infection with H. pylori 60190 but not CagA-deficient 60190 (60190ΔCagA). d DGC cells infected with H. pylori (60190) showed an increased migration rate, which was not observed after 60190ΔCagA infection. e Basal expression of CagA from MKN45 cells treated transfected with CagA-expressing vector (CagA-pSP65SRa). f HOTAIR expression was similar between MKN45 cells infected with H. pylori (60190) and 60190ΔCagA. g HOTAIR expression was unchanged by CagA-pSP65SRa. Estradiol increased HOTAIR expression by ~ twofold, whereas co-treatment with estradiol and CagA-pSP65SRa increased HOTAIR expression by ~ threefold. h Cells co-treated with estradiol and CagA-pSP65SRa showed higher expression of EMT and stemness markers than those treated with estradiol or CagA alone. EMT epithelial–mesenchymal transition, CagA cytotoxin-associated gene A, MLL3 mixed-lineage leukemia protein 3, ER estrogen receptor, DGC diffuse gastric cancer. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control
When MKN45 cells were infected with H. pylori (60190) and 60190ΔCagA, the expression of HOTAIR was unchanged (P = 0.2372 (control vs. 60190), P = 0.2995 (control vs. 60190ΔCagA), and P = 0.5379 (60190 vs. 60190ΔCagA), Fig. 3f). However, HOTAIR expression increased after co-treatment with the CagA-expressing vector and estradiol compared with transfection of the CagA-expressing vector alone (P = 0.2624 (vector vs. CagA-pSP65SRa), P = 0.0025 (CagA-pSP65SRa vs. CagA-pSP65SRa + E2), and P = 0.0018 (vector vs. CagA-pSP65SRa + E2), Fig. 3g), resulting in higher HOTAIR expression than that observed after estradiol treatment (Fig. 2b, ~ twofold increased expression after estradiol treatment vs. Figure 3g, ~ threefold increased expression after estradiol + CagA-pSP65SRa treatment).
In MKN45 cells transfected with CagA-expressing vector, the expression of Snail, Slug, Vimentin, Oct4, and C-Myc increased and the expression of Zo1 and E-cadherin decreased. When MKN45 cells transfected with CagA-expressing vector were treated with estradiol, the expression of EMT and stemness markers increased to a greater extent than in cells transfected with only CagA-expressing vector (Fig. 3h). These results indicate that co-treatment of MKN45 cells with estradiol and CagA-expression vector induces HOTAIR expression by significantly increasing EMT and stemness phenotype than treatment with estradiol or CagA-expression vector alone.
Estradiol enhances DGC organoid growth
We next performed gastric cancer organoid culture using human tumor tissue samples obtained by endoscopic biopsy that were histologically confirmed as poorly cohesive carcinoma. Out of DGC organoids from five DGC patients, three were ERα-positive (#1–3) and two were ERα-negative (#4, 5) (Fig. 4a). To determine whether estrogen affects the growth of DGC organoids, we measured the size of organoids beyond passage 5 after culture with estradiol for 7 days. When the size of ERα-positive was compared on the 4th and 7th days after estradiol treatment, the size of organoids increased by two- to sixfold suggesting that estrogen enhances the growth of ERα-positive organoids (P = 0.0023 (Mock vs. E2), P = 0.0014 (E2 vs. E2 + ICI 182,780) in day 4; P = 0.0128 (Mock vs. E2), P < 0.001 (E2 vs. E2 + ICI 182,780) in day 7, Fig. 4b; P < 0.001 (Mock vs. E2), P < 0.001 (E2 vs. E2 + ICI 182,780) in day 4; P < 0.001 (Mock vs. E2), P < 0.001 (E2 vs. E2 + ICI 182,780) in day 7, Fig. 4c; P < 0.001 (Mock vs. E2), P < 0.001 (E2 vs. E2 + ICI 182,780) in day 4; P < 0.001 (Mock vs. E2), P < 0.001 (E2 vs. E2 + ICI 182,780) in day 7, Fig. 4d). By contrast, the size of ERα-negative DGC organoids did not increase when compared on days 4 and 7 after estradiol treatment (Fig. 4e, f). Estradiol increased expression of HOTAIR, by two- to sixfold in ERα-positive organoids (P = 0.0010 (control vs. E2, patient #1), P = 0.0370 (control vs. E2, patient #2), P = 0.0032 (control vs. E2, patient #3), Fig. 4g), but not in ERα-negative organoids (Fig. 4h). We measured the expression level of MLL3 by estradiol in organoids. In ERα-positive DGC organoids, MLL3 expression was significantly increased by estradiol (P = 0.0227 (#1), P = 0.0053 (#2), Supplementary Fig. S2a), but in ERα-negative DGC organoids, the effect of estradiol was not significant (Supplementary Fig. S2b). We performed experiments on cell behavior including cell migration, and the expression of EMT and stemness markers in organoids. Estradiol increased migration rate of ERα-positive organoids (#1), which was reversed by co-treatment with ICI 182,780 (P = 0.0239 (Mock vs. E2), P = 0.0402 (E2 vs. E2 + ICI 182,780), Fig. 4i). In contrast, estradiol did not affect the migration of ERα-negative organoids (#4, Fig. 4j). After estradiol treatment in ERα-positive organoids, the expression of E-cad showed a tendency to decrease, and the expression of C-myc tended to be increased (Supplementary Fig. S2c, d), which was similar as shown in the cell lines (Fig. 1m–q). This trend was not observed in ERα-negative organoids (Supplementary Fig. S2e, f).
Estradiol promotes the growth of DGC organoids. a Fresh tumor samples were obtained from DGC patients. Three ERα-positive and two ERα-negative DGC organoids were confirmed by western blotting. b, c, d Estradiol increased the size of ERα-positive organoids, which was reversed by co-treatment with ICI 182,780. The size change of ERα-positive and -negative organoids was compared at 4 and 7 days after estradiol treatment. The size of ERα-positive organoids increased by two- to sixfold. e, f The size of ERα-negative DGC organoids was not changed by estradiol. g, h Estradiol increased expression of HOTAIR, by two- to sixfold in ERα-positive DGC organoids, but not in ERα-negative organoids. i Estradiol increased migration rate of ERα-positive organoids, which was reversed by co-treatment with ICI 182,780. j Estradiol did not affect the migration of ERα-negative organoids. DGC diffuse gastric cancer, ER estrogen receptor, HOTAIR HOX antisense intergenic RNA. *P < 0.05, **P < 0.01, ***P < 0.001 vs. control
ERα expression may be associated with tumor invasion, and correlates with HOTAIR expression in young female patients with DGC
Using qPCR, we found that ERα expression was higher in tumor tissue than in paired gastric normal mucosa obtained from 57 DGC patients (median 0.007 vs. 0.072, P < 0.001, Fig. 5a). In our clinical data, the expression of ERα in IGC samples (n = 44) was also higher in tumor tissues than in adjacent normal tissues. (P = 0.013, Fig. 5b). When comparing the IGC tumor tissues and the DGC tumor tissues, the expression level of ERα was significantly higher in the DGC tumor samples than those of IGC (P = 0.013, Fig. 5c). However, as we showed in Fig. 1, it should be noted that ERα expression in IGC cells did not affect cell viability, migration, and invasion upon estradiol treatment, unlike in DGC cells. Next, we compared ERα expression in normal mucosa and tumor tissue according to its depth of invasion in 34 female DGC patients. ERα expression in tumor tissue with serosal invasion (T4) was higher than that with no serosal invasion (T1–T3) (mean 0.058 vs. 0.104, P = 0.0314, Fig. 5d), suggesting that ERα expression is associated with tumor invasion. Among the 34 female DGC patients, 25 were younger than 51 years of age, which is usually regarded as premenopausal (range 32–51 years, mean 41.32 ± 5.91 years). In these 25 young female DGC patients, we confirmed that ERα expression in tumor tissue was higher than that in paired normal mucosa (median 0.004 vs. 0.072, P < 0.001, Fig. 5e). When comparing the tumor tissues of IGC and DGC patients, the expression of HOTAIR was significantly higher in tumor samples from DGC patients than those from IGC patients (P = 0.01, Fig. 5f). Furthermore, ERα expression was positively correlated with HOTAIR expression within the 25 young female DGC patients (r = 0.898, P < 0.0001, Fig. 5g).
Differential expression and clinical significance of ERα and HOTAIR in human DGC tissue. a ERα expression was higher in tumor tissue than in paired normal gastric mucosa from DGC patients (n = 57). b In IGC patients, ERα expression was also higher in tumor tissues than in the adjacent normal gastric tissues. c ERα expression is higher in DGC tumor tissues than in IGC tumor tissues. d ERα expression was higher in tumor tissue with serosal invasion (T4, defined according to 8th American Joint Committee on Cancer TNM staging) compared with tumor tissue without serosal invasion (T1–T3). e ERα expression level was higher in tumor tissue than in paired normal gastric mucosa from young female patients (n = 25). f The expression of HOTAIR was significantly higher in DGC tumor tissues than that of IGC. g Among young female patients, ERα expression was correlated with HOTAIR expression. h HOTAIR expression was higher in H. pylori-infected patients when their tumor tissue was ERα-positive (n = 23). i, j ERα and HOTAIR expression was much higher in tumor tissue from an H. pylori-infected postpartum DGC patient than other female patients. ER estrogen receptor, HOTAIR HOX antisense intergenic RNA, DGC diffuse gastric cancer, IGC intestinal gastric cancer
Higher HOTAIR expression in young female DGC patients with ERα-positive tumors
Among the 25 young female DGC patients, 23 had current H. pylori infections in their gastric mucosa, and HOTAIR expression was higher when ERα was present in their cancer tissue (P = 0.0170, Fig. 5h). We found very high expression of ERα and HOTAIR in a H. pylori-infected postpartum patient compared with the other female patients (Fig. 5i, 5j), consistent with the aggressive DGC tumor progression in pregnant women. In this patient, endoscopy showed advanced gastric cancer (Borrmann type 4), abdominal computed tomography showed advanced gastric cancer with peritoneal seeding (Krukenberg tumor that invaded both ovaries), and histological examination revealed signet ring cell carcinoma (diffuse-type gastric adenocarcinoma). The histology of gastric tissue of this patient showed the presence of H. pylori and rapid urease test was also positive. The patient underwent palliative chemotherapy but died 5 months after the diagnosis, exhibiting very rapid cancer progression.
Discussion
We found that the growth of DGC was promoted by estrogen-induced transcription of HOTAIR in the presence of ERα. This effect was potentiated by CagA secreted from H. pylori, which recruits coregulators of EREs, and resulted in increased cell migration, induction of EMT, and a stem cell-like phenotype. Previous studies showed that the presence of hormone receptors in hormone-dependent tumors, such as breast and prostate cancer, predicts a favorable prognosis when hormonal therapy is administered [33, 34]. However, the role of hormone receptors in tumors of non-target organs, including the stomach, remains largely unknown. Although estrogen is reported to have anti-cancer effects in some non-target organs, such as the liver and colon [35], more studies are needed to understand the mechanisms and clarify areas of controversy. Whereas experimental and epidemiological data suggest that estrogen exerts a protective effect against the induction of IGC [36], the results of our study seem to indicate an opposing effect of estrogen on DGC.
ERs are found in 2–30% of human gastric cancers, mainly in the poorly differentiated type [36]. A recent meta‐analysis suggests that tumoral expression of ERα predicts poor survival [37]. However, the clinical significance of ERs and estrogen‐dependent tumor growth in gastric cancer are unclear. In addition, the physiologic concentration of estradiol depends on patient sex and age. Estradiol level is very low (< 0.073 nM)) in prepubertal children, postmenopausal women, and patients receiving aromatase inhibitors. However, women of childbearing age who undergo menstruation have estradiol levels ranging from 0.110 to 2.293 nM, which can rise to 73.420 nM during pregnancy [38]. In the present study, we used 0.1 nM estradiol to treat DGC cell lines, which is within the physiologic range of premenopausal women. A large surge of estrogen in pregnant woman may explain the rapid and aggressive progression of DGC during pregnancy [39]. Consistently, we observed a surge in ERα and HOTAIR expression in DGC tissue from a female patient who had recently delivered a child.
Similar to other studies [40, 41], we found that estrogen enhanced cancer cell proliferation, migration, and invasion in the presence of ERα, which was associated with poor prognosis in DGC patients based on tumor stage. Treatment with ICI 182,780 reversed these changes. Fulvestrant with ovarian function suppression using goserelin is a promising option in premenopausal patients with metastatic breast cancer [42, 43]. To confirm the efficacy of fulvestrant in premenopausal women with ERα-positive DGC, further clinical studies are warranted. We also validated the effect of estrogen on DGC organoids, confirming that estrogen directly regulated oncogenic HOTAIR and promoted tumorigenesis in the presence of ERα.
There are several studies showing that HOTAIR promotes gastric carcinogenesis. Recent studies have suggested HOTAIR expression was found to be higher in gastric cancer tissues than in the paired normal gastric tissues [44,45,46]. High expression level of HOTAIR was significantly associated with LN metastasis, TNM stage, and invasion, suggesting that it could be used as a predictor of poor overall survival in gastric cancer patients [46]. In one study, by downregulating HOTAIR using siRNA, HOTAIR was involved in inhibiting apoptosis of cancer cells, suggesting a role in promoting invasiveness [47]. The expression of HOTAIR was an independent prognostic factor and risk factor for peritoneal metastasis in gastric cancer patients [48]. The expression of HOTAIR reportedly promotes EMT that helps peritoneal dissemination of scirrhous gastric cancer [45]. These are in line with our study in terms of increased expression of EMT and stemness markers upon high expression of HOTAIR.
The HOTAIR promoter has many transcription factor binding sites and contains estrogen response elements (EREs) that are targeted by the estrogen–estrogen receptor (ER) complex [49]. The presence of multiple EREs suggests that estrogen may play a critical role in the expression of HOTAIR. Bhan et al. showed that HOTAIR expression in ER-positive breast cancer cells was transcriptionally activated by estradiol and that ER was important for this activation [49, 50]. In our study, we showed that estradiol directly interacts with two ERE binding sites in HOTAIR promotor regions. In another study, estrogen-mediated HOTAIR transcription increased cell viability, migration, and invasion of endometrial cancer cells [51]. In addition, ER-coregulators are important because they can bind to the promoters of genes regulated by estradiol. The estradiol-dependent activation of HOTAIR can occur in a variety of forms, and the MLL family is known universal ER-coregulator, and MLL1 and MLL3 bind to the promoter of the HOTAIR gene in the presence of estradiol [52]. In our study, the expression of MLL1 and MLL3, coregulators of ER complex, was increased when cells were infected with H. pylori. Of note, MLL3 was only a coregulator of ER complex mediated through CagA, an oncoprotein secreted by H. pylori, which suggests that CagA-secreting H. pylori may enforce estradiol-ER binding resulting in more transcription of HOTAIR compared to absence of CagA-secreting H. pylori. Notably, MLL3 expression was also induced upon estrogen treatment in ERα-positive DGC organoids in our study. These may explain why HOTAIR was expressed higher when cells were co-treated with estradiol and CagA-pSP65SRa than treated with estradiol alone.
H. pylori is a well-known carcinogen in gastric cancer. However, its carcinogenic mechanism is mostly understood in the context of IGC rather than DGC. We found that the migration abilities of DGC cells increased after infection with CagA-positive H. pylori but not after infection with CagA-deficient H. pylori. In addition, treatment with CagA (from H. pylori or vectors) increased the expression of EMT-related markers in DGC cells. Apart from these direct carcinogenic effects on cancer cells, CagA recruited MLL3, a coregulator of ER complex, in the transcriptional regulation of HOTAIR. Therefore, H. pylori may affect tumorigenesis of DGC in various ways.
Surprisingly, when a DGC cell line was treated with estradiol and CagA-expressing vector, the induction of EMT and stemness markers was further enhanced. In line with a previous study showing that HOTAIR expression is increased in gastric cancer tissue [53], we showed that ERα expression was positively correlated with HOTAIR expression. In addition, young female DGC patients with current H. pylori infection and ERα-positive tumor tissue showed increased expression of HOTAIR. We also observed much higher ERα and HOTAIR expression in cancer tissue from a postpartum patient than that from non-postpartum premenopausal women. Given the estrogen surge during pregnancy and current H. pylori infection of this patient, we presume that the synergistic effect of estrogen and H. pylori may have led to a high expression of HOTAIR and rapid cancer progression. Consistently, some cases of DGC progress very aggressively during pregnancy and have a very poor prognosis [36, 39, 54]. Taken together, these findings suggest that in ERα-positive DGC, when MLL3, a coregulator of HOTAIR transcription, is recruited by CagA secreted from H. pylori, estrogen attaches to the ERE of the HOTAIR promoter, thereby increasing the expression of oncogenic HOTAIR and promoting the progression of gastric cancer.
Patients without atrophic gastritis and a higher antibody titer to H. pylori have a higher risk of DGC, whereas those with atrophic gastritis and a lower antibody titer to H. pylori have a higher risk of IGC [55, 56]. This suggests that DGC develops more quickly or that more immunological reactions occur during the development of DGC compared with IGC. We found that both H. pylori CagA and estrogen drive DGC in young women. These findings may explain the 1:1 male-to-female ratio of gastric cancer among patients under 40 years of age in contrast to the 3:1 male-to-female ratio of gastric cancer overall [57]. In patients with IGC, ERα was more expressed in tumor samples than in adjacent normal tissue. In addition, in some IGC cell lines, ERα was highly expressed. However, estradiol addition to IGC cell lines with ERα expression did not make cells more viable, migratory or invasive, which suggests different role of ERα between DGC and IGC.
Our study has some limitations. First, as most patients were H. pylori-positive, we could not compare H. pylori-positive and -negative groups. However, in the clinical setting, the positive H. pylori rate in young DGC patients is very high—above 90%, and H. pylori has a more prominent role in tumor formation than it does among older patients [58]. Here, we show that HOTAIR expression was higher in ERα-positive patients with overt H. pylori infection than in ERα-negative patients, suggesting that estrogen and H. pylori work together to induce strong tumorigenesis in young women with DGC. Second, there was only one case of postpartum patients with DGC. However, such patient is rare in clinical setting and thus obtaining tumor tissue in the patient is much difficult. Lastly, when a DGC cell line was simultaneously infected with H. pylori and treated with estradiol, cells tended to become necrotic, so H. pylori infection was replaced with CagA-expressing vector transfection. However, as CagA secreted from H. pylori is a virulence factor that plays a key role in tumorigenesis, such replacement may be reasonable, and our study is the first to confirm the combined effect of CagA and estrogen in a gastric cancer cell line. In addition, we confirmed the combined effect of H. pylori and estrogen in human tissue.
In conclusion, estrogen may induce EMT and a stem cell phenotype in DGC cells and organoids by inducing transcription of the oncogenic HOTAIR via a synergistic effect of estrogen and infection with CagA-secreting H. pylori and recruitment of the ER complex coregulator MLL3. Thus, ICI 182,780, Fulvestrant, which is widely used to treat hormone receptor-positive breast cancer, could be considered a tumor-suppressive agent with ovarian function suppression in young female DGC patients in a premenopausal state. Further research would shed light on this possibility.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Ansari S, Gantuya B, Tuan VP, Yamaoka Y. Diffuse gastric cancer: a summary of analogous contributing factors for its molecular pathogenicity. Int J Mol Sci. 2018;19(8):2424.
Balakrishnan M, George R, Sharma A, Graham DY. Changing trends in stomach cancer throughout the world. Curr Gastroenterol Rep. 2017;19(8):36.
Henson DE, Dittus C, Younes M, Nguyen H, Albores-Saavedra J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: increase in the signet ring cell type. Arch Pathol Lab Med. 2004;128(7):765–70.
Tang C-T, Zeng L, Yang J, Zeng C, Chen Y. Analysis of the incidence and survival of gastric cancer based on the lauren classification: a large population-based study using SEER. Front Oncol. 2020;10:1212.
Lee JY, Gong EJ, Chung EJ, Park HW, Bae SE, Kim EH, et al. The characteristics and prognosis of diffuse-type early gastric cancer diagnosed during health check-ups. Gut Liver. 2017;11(6):807–12.
Ellison-Loschmann L, Sporle A, Corbin M, Cheng S, Harawira P, Gray M, et al. Risk of stomach cancer in Aotearoa/New Zealand: a Māori population based case-control study. PLoS ONE. 2017;12(7):e0181581.
Gong EJ, Lee JY, Bae SE, Park YS, Choi KD, Song HJ, et al. Characteristics of non-cardia gastric cancer with a high serum anti-Helicobacter pylori IgG titer and its association with diffuse-type histology. PLoS ONE. 2018;13(4):e0195264.
De B, Rhome R, Jairam V, Özbek U, Holcombe RF, Buckstein M, et al. Gastric adenocarcinoma in young adult patients: patterns of care and survival in the United States. Gastric Cancer. 2018;21(6):889–99.
Karat D, Brotherick I, Shenton BK, Scott D, Raimes SA, Griffin SM. Expression of oestrogen and progesterone receptors in gastric cancer: a flow cytometric study. Br J Cancer. 1999;80(8):1271–4.
Takano N, Iizuka N, Hazama S, Yoshino S, Tangoku A, Oka M. Expression of estrogen receptor-α and -β mRNAs in human gastric cancer. Cancer Lett. 2002;176(2):129–35.
Wang M, Pan JY, Song GR, Chen HB, An LJ, Qu SX. Altered expression of estrogen receptor alpha and beta in advanced gastric adenocarcinoma: correlation with prothymosin alpha and clinicopathological parameters. Eur J Surg Oncol. 2007;33(2):195–201.
Xu CY, Guo JL, Jiang ZN, Xie SD, Shen JG, Shen JY, et al. Prognostic role of estrogen receptor alpha and estrogen receptor beta in gastric cancer. Ann Surg Oncol. 2010;17(9):2503–9.
Ryu WS, Kim JH, Jang YJ, Park SS, Um JW, Park SH, et al. Expression of estrogen receptors in gastric cancer and their clinical significance. J Surg Oncol. 2012;106(4):456–61.
Group HaCC. Gastric cancer and Helicobacter pylori: a combined analysis of 12 case control studies nested within prospective cohorts. Gut. 2001;49(3):347–53.
Cho SJ, Choi IJ, Kim CG, Lee JY, Kook MC, Seong MW, et al. Helicobacter pylori seropositivity is associated with gastric cancer regardless of tumor subtype in Korea. Gut Liver. 2010;4(4):466–74.
Kwak HW, Choi IJ, Cho SJ, Lee JY, Kim CG, Kook MC, et al. Characteristics of gastric cancer according to Helicobacter pylori infection status. J Gastroenterol Hepatol. 2014;29(9):1671–7.
Kishikawa H, Kimura K, Takarabe S, Kaida S, Nishida J. Helicobacter pylori antibody titer and gastric cancer screening. Dis Markers. 2015;2015:156719.
Cho SJ, Choi IJ, Kook MC, Nam BH, Kim CG, Lee JY, et al. Staging of intestinal- and diffuse-type gastric cancers with the OLGA and OLGIM staging systems. Aliment Pharmacol Ther. 2013;38(10):1292–302.
Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group and Review Pannel. Korean practice guideline for gastric cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019;19(1):1–48.
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–9.
Ferrand J, Noël D, Lehours P, Prochazkova-Carlotti M, Chambonnier L, Ménard A, et al. Human bone marrow-derived stem cells acquire epithelial characteristics through fusion with gastrointestinal epithelial cells. PLoS ONE. 2011;6(5):e19569.
Cho SJ, Kook MC, Lee JH, Shin JY, Park J, Bae YK, et al. Peroxisome proliferator-activated receptor γ upregulates galectin-9 and predicts prognosis in intestinal-type gastric cancer. Int J Cancer. 2015;136(4):810–20.
Shin J-Y, Kim Y-I, Cho S-J, Lee MK, Kook M-C, Lee JH, et al. MicroRNA 135a suppresses lymph node metastasis through down-regulation of ROCK1 in early gastric cancer. PLoS ONE. 2014;9(1):e85205.
Cho SJ, Yoon C, Lee JH, Chang KK, Lin JX, Kim YH, et al. KMT2C Mutations in Diffuse-Type Gastric Adenocarcinoma Promote Epithelial-to-Mesenchymal Transition. Clin Cancer Res. 2018;24(24):6556–69.
Lee J, Park J, Park M, Na Y, Cho S-J. A Comparative study of Helicobacter pylori growth on different agar-based media. Korean J Helicobacter Upper Gastrointest Res. 2017;17:208.
Choi SI, Yoon C, Park MR, Lee D, Kook MC, Lin JX, et al. CDX1 expression induced by CagA-expressing Helicobacter pylori promotes gastric tumorigenesis. Mol Cancer Res. 2019;17(11):2169–83.
Fujii Y, Yoshihashi K, Suzuki H, Tsutsumi S, Mutoh H, Maeda S, et al. CDX1 confers intestinal phenotype on gastric epithelial cells via induction of stemness-associated reprogramming factors SALL4 and KLF5. Proc Natl Acad Sci U S A. 2012;109(50):20584–9.
Nanni S, Aiello A, Re A, Guffanti A, Benvenuti V, Colussi C, et al. Estrogen-dependent dynamic profile of eNOS-DNA associations in prostate cancer. PLoS ONE. 2013;8(5):e62522.
Hajjari M, Salavaty A. HOTAIR: an oncogenic long non-coding RNA in different cancers. Cancer Biol Med. 2015;12(1):1–9.
Pawłowska E, Szczepanska J, Blasiak J. The long noncoding RNA HOTAIR in breast cancer: does autophagy play a role? Int J Mol Sci. 2017;18(11):2317.
Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52(24):6735–40.
Hurvitz SA, Pietras RJ. Rational management of endocrine resistance in breast cancer: a comprehensive review of estrogen receptor biology, treatment options, and future directions. Cancer. 2008;113(9):2385–97.
Ceresoli GL, De Vincenzo F, Sauta MG, Bonomi M, Zucali PA. Role of chemotherapy in combination with hormonal therapy in first-line treatment of metastatic hormone-sensitive prostate cancer. Q J Nucl Med Mol Imaging. 2015;59(4):374–80.
Hua H, Zhang H, Kong Q, Jiang Y. Mechanisms for estrogen receptor expression in human cancer. Exp Hematol Oncol. 2018;7:24.
Jaspers VK, Gillessen A, Quakernack K. Gastric cancer in pregnancy: do pregnancy, age or female sex alter the prognosis? Case reports and review. Eur J Obstet Gynecol Reprod Biol. 1999;87(1):13–22.
Ge H, Yan Y, Tian F, Wu D, Huang Y. Prognostic value of estrogen receptor α and estrogen receptor β in gastric cancer based on a meta-analysis and The Cancer Genome Atlas (TCGA) datasets. Int J Surg. 2018;53:24–31.
Stanczyk FZ, Clarke NJ. Measurement of estradiol—challenges ahead. J Clin Endocrinol Metab. 2014;99(1):56–8.
Song MJ, Park YS, Song HJ, Park SJ, Ahn JY, Choi KD, et al. Prognosis of pregnancy-associated gastric cancer: an age-, sex-, and stage-matched case-control study. Gut Liver. 2016;10(5):731–8.
Tang W, et al. Expression of estrogen receptors and androgen receptor and their clinical significance in gastrtic cancer. Oncotarget. 2017;8(25):40765–77.
Zhang Y, Cong X, Li Z, Xue Y. Estrogen facilitates gastric cancer cell proliferation and invasion through promoting the secretion of interleukin-6 by cancer-associated fibroblasts. Int Immunopharmacol. 2020;78:105937.
Bartsch R, Bago-Horvath Z, Berghoff A, DeVries C, Pluschnig U, Dubsky P, et al. Ovarian function suppression and fulvestrant as endocrine therapy in premenopausal women with metastatic breast cancer. Eur J Cancer. 2012;48(13):1932–8.
Kim JY, Im SA, Jung KH, Ro J, Sohn J, Kim JH, et al. Fulvestrant plus goserelin versus anastrozole plus goserelin versus goserelin alone for hormone receptor-positive, HER2-negative tamoxifen-pretreated premenopausal women with recurrent or metastatic breast cancer (KCSG BR10-04): a multicentre, open-label, three-arm, randomised phase II trial (FLAG study). Eur J Cancer. 2018;103:127–36.
Hajjari M, Behmanesh M, Sadeghizadeh M, Zeinoddini M. Up-regulation of HOTAIR long non-coding RNA in human gastric adenocarcinoma tissues. Med Oncol. 2013;30(3):670.
Takei Y, Hara T, Suzuki A, Mihara K, Yanagihara K. Long noncoding RNA HOTAIR promotes epithelial-mesenchymal transition and is a suitable target to inhibit peritoneal dissemination in human scirrhous gastric cancers. Pathobiology. 2020;87(5):277–90.
Tang Q, Hann SS. HOTAIR: an oncogenic long non-coding RNA in human cancer. Cell Physiol Biochem. 2018;47(3):893–913.
Lee NK, Lee JH, Park CH, Yu D, Lee YC, Cheong JH, et al. Long non-coding RNA HOTAIR promotes carcinogenesis and invasion of gastric adenocarcinoma. Biochem Biophys Res Commun. 2014;451(2):171–8.
Okugawa Y, Toiyama Y, Hur K, Toden S, Saigusa S, Tanaka K, et al. Metastasis-associated long non-coding RNA drives gastric cancer development and promotes peritoneal metastasis. Carcinogenesis. 2014;35(12):2731–9.
Bhan A, Hussain I, Ansari KI, Kasiri S, Bashyal A, Mandal SS. Antisense transcript long noncoding RNA (lncRNA) HOTAIR is transcriptionally induced by estradiol. J Mol Biol. 2013;425(19):3707–22.
Bhan A, Mandal SS. LncRNA HOTAIR: a master regulator of chromatin dynamics and cancer. Biochim Biophys Acta. 2015;1856(1):151–64.
Zhou YX, Wang C, Mao LW, Wang YL, Xia LQ, Zhao W, et al. Long noncoding RNA HOTAIR mediates the estrogen-induced metastasis of endometrial cancer cells via the miR-646/NPM1 axis. Am J Physiol Cell Physiol. 2018;314(6):C690-c701.
Bhan A, Mandal SS. Estradiol-induced transcriptional regulation of long non-coding RNA. HOTAIR Methods Mol Biol. 2016;1366:395–412.
Xu Z, Chen H, Yang B, Liu X, Zhou X, Kong H. The association of HOTAIR with the diagnosis and prognosis of gastric cancer and its effect on the proliferation of gastric cancer cells. Can J Gastroenterol Hepatol. 2019;2019:3076345.
Maggen C, Lok CA, Cardonick E, van Gerwen M, Ottevanger PB, Boere IA, et al. Gastric cancer during pregnancy: a report on 13 cases and review of the literature with focus on chemotherapy during pregnancy. Acta Obstet Gynecol Scand. 2020;99(1):79–88.
Tatemichi M, Sasazuki S, Inoue M, Tsugane S. Clinical significance of IgG antibody titer against Helicobacter pylori. Helicobacter. 2009;14(3):231–6.
Majima A, Handa O, Naito Y, Dohi O, Okayama T, Yoshida N, et al. Early-stage gastric cancer can be found in improved atrophic mucosa over time from successful Helicobacter pylori eradication. Digestion. 2017;95(3):194–200.
Li J. Gastric cancer in young adults: a different clinical entity from carcinogenesis to prognosis. Gastroenterol Res Pract. 2020;202:9512707.
Huang JQ, Sridhar S, Chen Y, Hunt RH. Meta-analysis of the relationship between Helicobacter pylori seropositivity and gastric cancer. Gastroenterology. 1998;114(6):1169–79.
Acknowledgements
This study was supported by a grant from the National Research Foundation of Korea (#NRF-2019R1A2C1009923, #NRF-2022R1A2B5B01001430), the Korean Gastroenterology Fund for Future Development (2018), Seoul National University College of Medicine (grant no. 800-20200465) and the Korean College of Helicobacter and Upper Gastrointestinal Research Foundation (KCHUGR—202002001). 293T cell lines producing R-spondin1 and Noggin were kindly provided by Hubrecht Institute, The Netherlands. The authors thank Yong Chan Lee for providing the H. pylori (60190) and the full-length CagA gene of H. pylori standard strain NCTC1167.
Author information
Authors and Affiliations
Contributions
Seungkyung Kang and Miree Park have contributed equally to this work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For analyses using gastric cancer tissue, informed written consent was obtained from all patients, and the study was exempted from review by the Institutional Review Board of the National Cancer Center of Korea because it involved minimal risk due to the use of existing specimens (NCCTTR-17007). For organoid culture, the procedure was approved by the Institutional Review Board of Seoul National University Hospital (IRB #1901-166-1007 & #H-2203-009-1303).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10120_2022_1290_MOESM2_ESM.docx
Supplementary Fig. S1 a, b There was no significant change in the expression of EMT markers Snail and Slug in estradiol-treated AGS cells (ERα-negative IGC cell line) and SNU216 cells (ERα-positive IGC cell line). c, d In both IGC cell lines, estradiol did not affect the expression of stemness markers, C-myc and Oct4. EMT epithelial mesenchymal transition, IGC intestinal gastric cancer, ER estrogen receptor
10120_2022_1290_MOESM3_ESM.docx
Supplementary Fig. S2 a, b In ERα-positive DGC organoids, MLL3 expression was significantly increased by estradiol, but not in ERα-negative DGC organoids. c, d In estradiol treated ERα-positive organoids, the EMT marker E-cad showed a tendency to decrease, and the stemness marker C-myc showed a tendency to increase. e, f This tendency was not observed in ERα-negative organoids. ER estrogen receptor, IGC intestinal gastric cancer, EMT epithelial mesenchymal transition. *P < 0.05, **P < 0.01, ***P<0.001 vs. control
Rights and permissions
About this article
Cite this article
Kang, S., Park, M., Cho, J.Y. et al. Tumorigenic mechanisms of estrogen and Helicobacter pylori cytotoxin-associated gene A in estrogen receptor α-positive diffuse-type gastric adenocarcinoma. Gastric Cancer 25, 678–696 (2022). https://doi.org/10.1007/s10120-022-01290-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-022-01290-0