Abstract
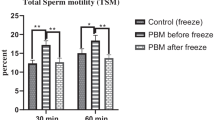
Low-level laser therapy (LLLT) can modulate redox state of the cell which could be useful to treat testicular degeneration and also prevent injuries by sperm cryopreservation. The aim of this study was to evaluate the effects of LLLT treatment on semen cryopreservation from rams submitted or not to testicular degeneration by testicular insulation. Eleven White Dorper rams were divided into four groups: animals that were not insulated (Control) and not treated (No Laser) (n = 2); animals that were not insulated and treated with LLLT (n = 3); animals that were insulated and not treated with LLLT (n = 3), and animals that were insulated and treated with LLLT (n = 3). Testicular insulation was performed using scrotal insulation bags for 72 h. LLLT treatment was 28 J/cm2 energy, 808 nm of wavelength, and 30 mW of power output, irradiated on testis for 15 days with an interval of 48 h. Three ejaculates from each ram were collected: before insulation, 23, and 59 days after insulation bag removal. Cryopreservation was performed of the third ejaculate. Sperm evaluation was performed before and after cryopreservation considering sperm motility, morphology, acrosomal and plasma membrane integrity, mitochondrial potential, and oxidative stress. As expected, cryopreservation had a negative effect on several sperm motility characteristics and sperm membranes. LLLT treatment did not improve sperm quality from rams submitted to testicular insulation. Thus, testicular insulation and cryopreservation effects on spermatozoa were not attenuated by LLLT in this study.
Similar content being viewed by others
References
Hansen PJ (2009) Effects of heat stress on mammalian reproduction. Philos Trans R Soc Lond B Biol Sci 364:3341–3350. https://doi.org/10.1098/rstb.2009.0131
Paul C, Teng S, Saunders PTK (2009) A single , mild , transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes , which induces germ cell death 1. Biol Reprod 919:913–919. https://doi.org/10.1095/biolreprod.108.071779
Setchell BP (1998) The Parkes lecture. Heat and the testis. J Reprod Fertil 114:179–194
Guthrie HD, Welch GR (2012) Effects of reactive oxygen species on sperm function. Theriogenology 78:1700–1708. https://doi.org/10.1016/j.theriogenology.2012.05.002
Dadarwal D, Mapletoft RJ, Adams GP, Pfeifer LF, Creelman C, Singh J (2013) Effect of progesterone concentration and duration of proestrus on fertility in beef cattle after fixed-time artificial insemination. Theriogenology 79:859–866. https://doi.org/10.1016/j.theriogenology.2013.01.003
Brito LF, Silva AE, Barbosa RT, Unanian MM, Kastelic JP (2003) Effects of scrotal insulation on sperm production, semen quality, and testicular echotexture in Bos indicus and Bos indicus x Bos taurus bulls. Anim Reprod Sci 79:1–15
Rahman MB, Vandaele L, Rijsselaere T, Maes D, Hoogewijs M, Frijters A, Noordman J, Granados A, Dernelle E, Shamsuddin M, Parrish JJ, Van Soom A (2011) Scrotal insulation and its relationship to abnormal morphology, chromatin protamination and nuclear shape of spermatozoa in Holstein-Friesian and Belgian blue bulls. Theriogenology 76:1246–1257. https://doi.org/10.1016/j.theriogenology.2011.05.031
Alves MBR, Andrade AFC, Arruda RP, Batissaco L, Florez-Rodriguez SA, Oliveira BMM, Torres MA, Lançoni R, Ravagnani GM, Prado Filho RR, Vellone VS, Losano JDA, Franci CR, Nichi M, Celeghini ECC (2016) Recovery of normal testicular temperature after scrotal heat stress in rams assessed by infrared thermography and its effects on seminal characteristics and testosterone blood serum concentration. Theriogenology 86:795–805.e2. https://doi.org/10.1016/j.theriogenology.2016.02.034
Kastelic JP, Cook RB, Coulter GH (1997) Scrotal/testicular thermoregulation and the effects of increased testicular temperature in the bull. Vet Clin North Am Food Anim Pract 13:271–282
Oristaglio Turner RM (2007) Pathogenesis, diagnosis, and management of testicular degeneration in stallions. Clin Tech Equine Pract 6:278–284. https://doi.org/10.1053/j.ctep.2007.09.006
Arruda RP, Silva DF, Alonso MA, Nascimento J, Oliveira LZ, Celeghini ECC, Affonso FJ, Martins SMMK (2010) Nutraceuticals in reproduction of bulls and stallions. Rev Bras Zootec 39:393–400
Santiani A, Evangelista S, Sepúlveda N, Risopatrón J, Villegas J, Sánchez R (2014) Addition of superoxide dismutase mimics during cooling process prevents oxidative stress and improves semen quality parameters in frozen/thawed ram spermatozoa. Theriogenology 82(6):884–889. https://doi.org/10.1016/j.theriogenology.2014.07.002
Partyka A, Nizanski W, Lukaszewicz E (2010) Evaluation of fresh and frozen-thawed fowl semen by flow cytometry. Theriogenology 74:1019–1027. https://doi.org/10.1016/j.theriogenology.2010.04.032
Curry MR, Millar JD, Watson PF (1994) Calculated optimal cooling rates for ram and human sperm cryopreservation fail to conform with empirical observations. Biol Reprod 51:1014–1021
Celeghini ECC, Arruda RP, De Andrade AFC, Nascimento J, Raphael CF, Rodrigues PHM (2008) Effects that bovine sperm cryopreservation using two different extenders has on sperm membranes and chromatin. Anim Reprod Sci 104:119–131. https://doi.org/10.1016/j.anireprosci.2007.02.001
Anzar M, He L, Buhr MM et al (2002) Sperm apoptosis in fresh and cryopreserved bull semen detected by flow cytometry and its relationship with fertility. Biol Reprod 66:354–360
Watson PF (2000) The causes of reduced fertility with cryopreserved semen. Anim Reprod Sci 60–61:481–492
Bjordal JM, Couppe C, Chow RT et al (2003) A systematic review of low level laser therapy with location-specific doses for pain from chronic joint disorders. Aust J Physiother 49:107–116
Peplow PV, Chung T-Y, Baxter GD (2010) Laser photobiomodulation of proliferation of cells in culture: a review of human and animal studies. Photomed Laser Surg 28(Suppl 1):S3–S40. https://doi.org/10.1089/pho.2010.2771
AlGhamdi KM, Kumar A, Moussa NA (2012) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27:237–249. https://doi.org/10.1007/s10103-011-0885-2
Lubart R, Eichler M, Lavi R et al (2005) Low-energy laser irradiation promotes cellular redox activity. Photomed Laser Surg 23:3–9. https://doi.org/10.1089/pho.2005.23.3
Alves MBR, de Arruda RP, Batissaco L et al (2016) Low-level laser therapy to recovery testicular degeneration in rams: effects on seminal characteristics, scrotal temperature, plasma testosterone concentration, and testes histopathology. Lasers Med Sci 31:695–704. https://doi.org/10.1007/s10103-016-1911-1
Taha MF, Valojerdi MR (2004) Quantitative and qualitative changes of the seminiferous epithelium induced by Ga. Al. As. (830 nm) laser radiation. Lasers Surg Med 34:352–359. https://doi.org/10.1002/lsm.20027
Blom E (1973) The ultrastructure of some characteristic sperm deffects and a proposal for a new classification of the bull spermiogram. Nord Vet Med 25:383–339
Celeghini ECC, Nascimento J, Raphael CF et al (2010) Simultaneous assessment of plasmatic, acrosomal, and mitochondrial membranes in ram sperm by fluorescent probes. Arq Bras Med Vet Zootec 62:536–543
Alves MBR, De Andrade AFC, Arruda RP, Batissaco L, Florez- Rodriguez SA, Lançoni R, Oliveira BMM, Torres MA, Ravagnani GM, Almeida TG, Vellone VS, Celeghini ECC (2015) An efficient technique to detect sperm reactive oxygen species: the CellRox deep red® fluorescent probe. Biochem Physiol open access. https://doi.org/10.4172/2168-9652.1000157
Arman C, Quintana Casares PI, Sanchez-Partida LG, Setchell BP (2006) Ram sperm motility after intermittent scrotal insulation evaluated by manual and computer-assisted methods. Asian J Androl 8:411–418. https://doi.org/10.1111/j.1745-7262.2006.00145.x
Cruz Júnior CA, Lucci CM, Peripolli V, Silva AF, Menezes AM, Morais SRL, Araújo MS, Ribeiro LMCS, Mattos RC, McManus C (2015) Effects of testicle insulation on seminal traits in rams: preliminary study. Small Rumin Res 130:157–165. https://doi.org/10.1016/j.smallrumres.2015.06.014
Kopeika J, Thornhill A, Khalaf Y (2015) The effect of cryopreservation on the genome of gametes and embryos: principles of cryobiology and critical appraisal of the evidence. Hum Reprod Update 21:209–227. https://doi.org/10.1093/humupd/dmu063
Gao D, Critser JK (2000) Mechanisms of cryoinjury in living cells. ILAR J 41:187–196
Watson PF, Duncan AE (1988) Effect of salt concentration and unfrozen water fraction on the viability of slowly frozen ram spermatozoa. Cryobiology 25:131–142
Yeste M (2016) Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 85:47–64. https://doi.org/10.1016/j.theriogenology.2015.09.047
Meyers SA (2016) Spermatozoal response to osmotic stress. Anim Reprod Sci 89:57–64. https://doi.org/10.1016/j.anireprosci.2005.06.026
Watson PF (1995) Recent developments and concepts in the cryopreservation of spermatozoa and the assessment of their post-thawing function. Reprod Fertil Dev 7:871–891
Salamon S, Maxwell WMC (1995) Frozen storage of ram semen II. Causes of low fertility after cervical insemination and methods of improvement. Anim Reprod Sci 38:1–36. https://doi.org/10.1016/0378-4320(94)01328-J
Salamon S, Maxwell WM (2000) Storage of ram semen. Anim Reprod Sci 62:77–111
Hamilton TRDS, Mendes CM, de Castro LS, Assis PM, Siqueira AFP, Delgado JC, Goissis MD, Muiño-Blanco T, Cebrián-Pérez JA, Nichi M, Visintin JA, Assumpção MEOA (2016) Evaluation of lasting effects of heat stress on sperm profile and oxidative status of ram semen and epididymal sperm. Oxidative Med Cell Longev 2016:1687657. https://doi.org/10.1155/2016/1687657
Wardlaw JL, Sullivan TJ, Lux CN, Austin FW (2012) Photodynamic therapy against common bacteria causing wound and skin infections. Vet J 192:374–377. https://doi.org/10.1016/j.tvjl.2011.09.007
Salman Yazdi R, Bakhshi S, Jannat Alipoor F, Akhoond MR, Borhani S, Farrahi F, Lotfi Panah M, Sadighi Gilani MA (2013) Effect of 830-nm diode laser irradiation on human sperm motility. Lasers Med Sci 29:97–104. https://doi.org/10.1007/s10103-013-1276-7
Corral-Baqués MI, Rivera MM, Rigau T, Rodríguez-Gil JE, Rigau J (2008) The effect of low-level laser irradiation on dog spermatozoa motility is dependent on laser output power. Lasers Med Sci 24:703–713. https://doi.org/10.1007/s10103-008-0606-7
Ocaña-Quero JM, Gomez-Villamandos R, Moreno-Millan M, Santisteban-Valenzuela JM (1997) Biological effects of helium-neon (He-Ne) laser irradiation on acrosome reaction in bull sperm cells. J Photochem Photobiol B Biol 40:294–298. https://doi.org/10.1016/S1011-1344(97)00072-9
Fernandes GHC, de Carvalho Pde TC, Serra AJ, Crespilho AM, Peron JP, Rossato C, Leal-Junior EC, Albertini R (2015) The effect of low-level laser irradiation on sperm motility, and integrity of the plasma membrane and acrosome in cryopreserved bovine sperm. PLoS One 10:e0121487. https://doi.org/10.1371/journal.pone.0121487
Dobrin N, Zamfirescu S, Angehel AH, Topoleanu I, Nicolaia I, Paventi G, Coprean D (2015) Study on the effects of exposure to different doses of energy generated by a He-Ne laser on the quality of frozen-thawed semen of ram. Rom Biotechnol Lett 20:10381–10387
Ibrahim MAR, Zamfirescu S, Angehel A, Dobrin N, Abdelrazek I, El-Sharawy ME, El-Seviy E-S, Mocuta D (2014) The effects of helium-neon laser with different energy doses on cryopreserved ram semen quality in vitro examination. Scientific Papers Series D Animal Sci 42:149–152
Bermudez D, Carrasco F, Diaz F, Perez-de-Vargas I (1991) Germ cell DNA quantification shortly after IR laser radiation. Andrologia 23:303–307
Bermúdez D, Carrasco F, Pérez de Vargas I (1993) Effect of IR laser radiation on germ cell DNA content after one cycle of the seminiferous epithelium. Arch Androl 31:177–181. https://doi.org/10.3109/01485019308988397
Acknowledgements
The authors thank Mr. João Carlos Pinto de Campos, Mr. Márcio Donizete De Carli, and Mr. José Maria Bernardi for assistance to the animals.
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) by scholarship to Tamie Guibu de Almeida (process number 2013/13438-4) and by financial support to research to Eneiva Carla Carvalho Celeghini (process number 2012/00040-0), head of research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
The experiment agrees with ethical principles in animal research adopted by “Ethic Committee in the Use of Animals” of the School of Veterinary Medicine and Animal Science of University of São Paulo, protocol number 2467/2012.
Rights and permissions
About this article
Cite this article
de Almeida, T.G., Alves, M.B.R., Batissaco, L. et al. Does low-level laser therapy on degenerated ovine testes improve post-thawed sperm characteristics?. Lasers Med Sci 34, 1001–1009 (2019). https://doi.org/10.1007/s10103-018-2690-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-018-2690-7