Abstract
This study investigates the potential of hydroalcoholic extracts of Cistus ladanifer L., Erica Andevalensis and Rubus idaeus L. as a green method for the recovery of platinum group metals (PGMs) from both synthetic unimetallic solutions and multimetallic solutions obtained from the leaching of two different spent automotive catalytic converters (SACC). Experiments with unimetallic solutions revealed that E. andevalensis and R. idaeus extracts could separate about 70% of Pd and less than 40% of other tested metals (Al, Ce, Fe and Pt) from the solutions. Then, application of the plant extracts to two different SACCs leachates showed that E. andevalensis and R. idaeus extracts can induce high precipitation (> 60%) of Pd and Pt with co-precipitation of less than 20% of other metals. UV–Visible spectra analysis confirmed the bio-reduction of Pd2+ ions into Pd0 nanoparticles by R. idaeus extract, and Fourier transform infrared spectroscopy (FTIR) analysis revealed the contribution of functional groups of the phytochemicals present in the extract (such as phenols, flavonoids and anthocyanins) in the Pd2+ bio-reduction and stabilization. Afterward, scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM–EDX) analysis of the precipitate obtained from one leachate with R. idaeus extract demonstrated the presence of Pd particles along with organic compounds and particles containing other metals. Therefore, particles were subjected to a washing step with acetone for further purification. Finally, scanning transmission electron microscopy with energy-dispersive X-ray spectroscopy (STEM-EDX) analysis showed the high purity of the final Pd particles and high-resolution STEM allowed to determine their size variation of 2.5 to 17 nm with an average Feret size of 6.1 nm and confirmed their crystalline structure with an interplanar lattice distance of ~ 0.22 nm. This green approach offers various benefits including simplicity of Pd separation from the leachates as valuable nanoparticles that makes the process more feasible from economic and environmental standpoints. A process cost of ~ 20 $/g of Pd particles recovered was estimated (excluding manpower).
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last century, due to rapid industrialization, the application of platinum group metals (PGMs) emerged in various industrial sectors. The demand for PGMs is continuously increasing and more than 90% of their applications in the industries are in the catalysts’ processing (Wongsawa et al. 2020). The rising demand for these metals is reported to be at a higher pace than the other base metals used primarily in catalysts (Wei et al. 2019).
Automotive catalytic converters (ACC) have been increasingly applied for the transformation of carbon monoxide and hydrocarbons into carbon dioxide and water since the mid-1970 (Benson et al. 2000). Every year, a considerable part of the global extraction of PGMs is applied in ACC production, comprising 30% of Pt, 80% of Pd and Rh (Supply Chain Deep Dive Assessment 2022). Thus, SACCs that are replaced or from automotives that reach the end of their life become a secondary source of these metals. PGM grades in the ores range from 3 to 8 g/t (Kyriakakis 2005), while the monoliths of ACCs contain PGMs in a range of 1–3 kg/t (Karim and Ting 2021). Therefore, PGM recovery from SACCs reduces the energy consumption, waste disposals and environmental pollutants. For example, Fornalczyk and Saternus (2011) reported that in order to obtain 1 kg of Pt, about 150 tons of natural ores needs to be processed, which generates 400 tons of wastes, while the recycling of the same amount of Pt can be achieved by processing of 2 tons of ACCs. From 2008 to 2018, demand for PGMs has been raised about 10%, and they are mostly used in the catalytic converter industry (Platinum 2008; PGM Market Report 2018). In fact, it is expected that due to more strict environmental regulations, the demand trend for PGMs will continuously increase (Saguru et al. 2018). Considering this increasing secondary source of PGMs in ACCs, recycling these metals is critical from both financial and environmental standpoints. Recycling and long-term management of these wastes are critical steps to ensure the long-term viability of mining resources, thus mitigating the rising resource scarcity. However, due to the low concentration and high metal complexity in these wastes, their recycling still remains a challenge (Zhang and Xu 2018).
Currently for the metal recovery processes, hydrometallurgical methods are favored over pyrometallurgical methods and are developing every day since they are more environmentally and economically viable, with possibility of performing in wide-scale, diverse reagent options and using leachates from materials containing very low to very high grade of metals (Jeon et al. 2020; Kumari and Samadder 2022). PGM hydrometallurgical recovery from wastes starts with a metal leaching procedure (typically nonmetal-specific) and is followed by a metal separation phase from the generated leachates (Karim and Ting 2021). In the former conventional and long-established leaching systems for the recovery of PGMs, highly toxic and/or aggressive leaching solutions are employed, such as cyanide-containing media (with ~ 5% to ~ 20% cyanide, at ~ 100 °C to ~ 180 °C) or aqua regia (~ 12 M of acidity, at ~ 70 °C to ~ 110 °C) (Sibrell et al. 1994; Jafarifar et al. 2005; Chen and Huang 2006; Baghalha et al. 2009; Yakoumis et al. 2021). However, more recently the mainstream processes employ HCl-based systems with H2O2 as oxidizing agent, which are known to be greener than other leaching systems and still have PGM recoveries of about 95% (Jimenez de Aberasturi et al. 2011). The addition of oxidizing agents improves the hydrolysis efficiency and reduces the pollutant gases in the leaching system, allowing to decrease the acidity by balancing the ratio of HCl to H2O and/or using other oxidizing and chlorine sources including AlCl3, NaCl, NaClO and CuCl2 (Yakoumis et al. 2021). Regarding economic aspects, the interests of the concerned industries were initially mainly focused on the recovery efficiency; nevertheless, more recently the focus is also on developing sustainable processes with less environmental impact (Trinh et al. 2020). In the leaching step, which is the most crucial stage in hydrometallurgy, mild leaching procedures (lower acidities, temperatures and leaching times) are known to decrease waste generation, thus lowering the waste management and operational costs. For instance, mild HCl–H2O2-based leaching systems (generally using ~ 3 M to 6 M HCl and 1% to 10% H2O2 at 25 to 90 °C for ~ 3 h) do not emit toxic by-products and gases, therefore being among the most efficient and sustainable processes for the recovery of PGMs from SACCs (Yakoumis et al. 2021).
Different physiochemical methods for PGM separation from the leaching solutions are described in the literature, such as solvent extraction (Costa et al. 2013; Paiva et al. 2022), ion exchange (Lanaridi et al. 2022), molecular recognition technology (Izatt et al. 2015) and molecular ion imprinted polymer (Limjuco and Burnea 2022). However, some of those techniques may have drawbacks such as inadequate metal recovery (especially for low concentrations of metals), high energy requirements, as well as high chemical costs and environmental problems. This issue leaves open space for the emergence of new technologies aiming efficient PGM recovery with less operational costs and reduction in energy consumption while being environmentally friendly (Granados-Fernández et al. 2021). As a result, metal bio-recovery strategies, which make use of biological materials and/or processes, have attracted growing attention in recent years.
Recovery of PGMs from ACCs through bio-hydrometallurgical methods have not been extensively studied yet, which shows the importance of further investigations into alternative sustainable and effective PGM recovery processes with a low-carbon footprint (Karim and Ting 2021). To the best of our knowledge, by now, studied bio-hydrometallurgical procedures are mainly focused on the PGM bio-leaching from ACCs, benefiting from different organic acids or microorganisms. Bio-leaching processes employ organic acids such as oxalic or lactic acids (Wiecka et al. 2022) or microorganisms including bacteria, such as reported by Ilyas et al. (2022) and Karim and Ting (2022), or fungus like reported by Bahaloo-Horeh and Mousavi (2022). Nevertheless, it has been shown that microorganisms can also be used to separate PGMs from solutions by different mechanisms, including bio-reduction, bio-precipitation, chelation, extracellular sequestration and bio-sorption (Zhang et al. 2020). Moreover, it is known that several plants’ secondary metabolites can interact with metal ions through different mechanisms. Plants synthesize a wide variety of bioactive compounds, including phenols, saponins, alkaloids, organic acids, proteins, etc., which possess polar functional groups such as phenolic, hydroxyl, carboxyl, amino and sulfo, which capacitates these compounds to interact with metals through different processes, such as reduction (Ishak et al. 2019) and chelate/complex formation and precipitation (Ma et al. 2016). Thus, those compounds have application potential in various metal related industrial sectors, such as metal recovery/removal from solutions, biosynthesis of nanoparticles, treatment of metal-bearing wastewaters (Nobahar et al. 2021). In the case of PGMs, Ishak et al (2019) reviewed different studies about their interaction with plant compounds, pointing phenols, flavonoids, terpenoids, carbohydrates, proteins, amino acids and polysaccharides as the most important secondary metabolites having interaction potential with metals of this group. In fact, there are different works reporting the application of different secondary plant metabolite-rich extracts with PGM ions and their separation potential from unimetallic solutions as nanoparticles. For instance, Areca Nut Husk extracts (Hegde et al. 2021), Tea polyphenols (Hu et al. 2022) and Aspalathus linearis (Ismail et al. 2017) natural plant extracts are reported to separate successfully Pd, Pt and Rh ions respectively, from unimetallic solutions as nanoparticles.
In short, being aware that hydrometallurgical processes are advantageous compared to pyrometallurgical processes, to recover PGMs from ceramic honeycombs of SACCs it is necessary to decide which type of leaching process to apply. The choice between an approach based on toxic conditions and/or with very aggressive acidic solutions, or an approach with non-toxic and milder acidity solutions with an oxidizing agent, should in our opinion point to the second option, considering current concerns on waste management and environmental protection. Then, knowing that leaching is a nonmetal-specific process, it is necessary to decide how to recover PGMs from the leaching solution. At this point several methods allowing a high degree of selectivity to recover PGMs with great purity are possible, but require chemicals that may have high prices and/or be toxic and/or require the use of toxic solvents. On the other hand, the use of plant extracts to recover PGMs in a process known as nanoparticle green synthesis seems also possible. However, although this so-called green process has been widely studied with different types of plant materials, to the best of our knowledge these studies have always been carried out with unimetallic solutions. Thus, it seems that there is lack of studies on the application of plant extracts to recover target metals from multimetallic leaching solutions obtained from real waste materials.
The present work investigates a novel process in PGMs’ separation from unimetallic solutions and from multimetal-bearing leachates using three plant-based extracts and then studies the precipitates obtained from one leachate with a selected extract, aiming to contribute for the development of eco-friendly and efficient green technologies as alternatives for the recovery of these metals from secondary sources. After a first set of experiments using plant extracts to separate PGMs from unimetallic solutions at relatively low acidity (0.02 M HCl), the same extracts were used to recover PGMs from multimetallic leachates. For that purpose, two SACCs leachates obtained in harsh conditions (11.6 M HCl, 1% H2O2 and 60 °C) in a previous work were diluted in water to obtain solutions similar to those produced in milder leaching processes (more economic and environmentally sustainable), and the diluted leachates were used to study the potential application of nanoparticle green synthesis to recover PGMs avoiding the fast hydrolysis of plant compounds that would occur in harsh solutions.
Materials and methods
Experimental approach
The capacity of 70% (v/v) ethanolic plant extracts to precipitate metals was tested on different metal-bearing aqueous solutions: (1) first on unimetallic solutions prepared from standards commercialized for metals analysis and (2) then on leachates from spent automobile catalytic converters. For that purpose, the plant extracts were mixed and homogenized at a 1/1 (v/v) ratio with the metal-bearing solutions and mixtures prepared in the same way but using pure 70% ethanol (without plant compounds) were used as controls. The initial and the final concentrations of metals in the mixture were used to calculate percentages of removal from solution.
In addition, the putative effect of pH changes on metal removals was evaluated by comparing the initial pH measured in the metal-bearing solutions, the pH values in the mixtures and the estimated lowest pH at which metal precipitation is expected to occur according to theoretical simulations on the Medusa-Hydra software (Puigdomenech 2015) for increasing pH values (− 1 to 14) in aqueous solutions using parameters that mimic the tested matrices.
Experiments were carried out in triplicates in 50-mL centrifuge tubes at room temperature (20 ± 3 °C), and samples were collected for pH measurements and for metal analysis after 1 and 48 h of reaction.
The significance of differences between means of different experimental treatments (test 1 h, test 48 h, control 1 h and control 48 h) were assessed by the single factor analysis of variance (ANOVA) considering a significance threshold level of 5%. Then, when ANOVA revealed significant differences among treatments, post hoc tests were carried out with Tukey Kramer's tests (also for 5% significance level).
Plant extracts
Three types of plant leaves were collected for this study: from Cistus ladanifer L. (crimson spot rockrose); from Erica Andevalensis Cabezudo & Rivera (a shrub growing in the Iberian Peninsula next to acidic mine waters contaminated with sulfate and metals); and from Rubus idaeus L. (red raspberry). The choice of plants for this work considered criteria such as being available in the region, being reported as metal accumulators and/or being plants (or parts of them) considered industrial or agricultural waste. C. ladanifer has developed several tolerance mechanisms that allow its adaptability to contaminated environments. The immobilization of metallic elements in roots and accumulation in senescent leaves are examples of these adaptability mechanisms and make this plant a promising species for phyto-stabilization of mining areas (Abreu et al. 2011; Santos et al. 2012, 2014, 2016). E. andevalensis is an endemic species of the Iberian pyrite Belt (IPB) (Cabezudo and Rivera 1980) that grows under extreme conditions of pH values between 3 and 4 and high metal contents, being able to accumulate Mn (Rossini-Oliva et al. 2018). It colonizes mine tailings and the bank sediments of water bodies contaminated with acid mine drainage (AMD), such as the Tinto and Odiel rivers in Spain and the channels and dams at the São Domingos mine in Portugal (Abreu et al. 2008; Monaci et al. 2011). R. idaeus is an agricultural crop widely cultivated in Asia, Europe and North America for its fruits, being its leaves a residue highly rich in phenolic compounds (Pantelidis et al. 2007; Wang et al. 2019).
Young and mature leaves were collected and immediately dried to remove the moisture in an INCU-Line oven (VWR international) at 45 °C until their weights stabilized. The dried leaves were then grinded into powder using an electric coffee grinder and the powder was mixed at a 10% (w/v) ratio with 70% (v/v) ethanol. Afterward, the mixture was sonicated using an ultrasonic bath FB15054 (Fisher Scientific, USA) for 1 h and homogenized by orbital shaking at 150 rpm for 16 h. Finally, the mixture was centrifuged at 2800 g for 5 min at room temperature and the supernatant was filtered using 310–150 mm qualitative filter paper (VWR international) in a vacuum system.
Unimetallic solutions
Five 100 mg/L unimetallic solutions was prepared by diluting in 0.02 M HCl the following metal standard solutions of 1000 mg/L to a 1/10 (v/v) ratio: Pd(NO3)2 in 0.5 M HNO3, Ce(NO3)3 in 5% HNO3, Al(NO3)3 in 0.5 M HNO3 and Fe(NO3)3 in 0.5 M HNO3 (from Merck Certipur, Germany), and PtCl2 in 5% HCl from (Sigma-Aldrich standard for AAS, EUA).
Leachates from spent Automobile Catalytic Converters (SACCs)
Leachates from two different spent SACCs were used: (1) a SACC from a Seat Ibiza 1995 with 23 years of use, henceforth I95 and (2) a SACC from a Honda Civic 1998, with 20 years of use, henceforth H98. The leachates were provided by Professor Ana Paula Paiva from Faculdade de Ciências—Universidade de Lisboa (FCUL) and were prepared according to a process selected after several leaching experiments reported by Paiva et al. (2022). Both I95 and H98 metallic components were removed with a metal saw to extract the ceramic honeycombs loaded with the target catalytic metals. Then, the honeycombs were grind using a K, MF 10 Basic IKA Werke cutting mill at a speed of 3000 rpm with a 2.0 mm discharged grid. Afterward, leaching was carried out using the following conditions: [HCl] = 11.6 M, oxidizer H2O2 = 1% (w/v), liquid/solid = 2 L/kg, temperature = 60 °C, time = 3 h and stirring = 250 rpm. The HCl concentration of 11.6 M is due to the use of concentrated acid (37 wt% or 12 M), which is slightly diluted by the addition of 1% (w/v) oxidizer H2O2. Both leaching solutions were then diluted 1/6 (v/v) in demineralized water, reaching ~ 2 M HCl, to avoid the fast digestion of bio-compounds in the tests using plant extracts.
Analytical procedures
To measure the pH, a pH Meter GLP 21 (Crison, Spain) with a glass electrode (VWR, pH electrode SJ223) was used. For metal analysis on the mixtures of metal-bearing solutions with plant extracts, 2 mL samples was centrifuged at 2800 g for 5 min at room temperature (Hettich, ROTOFIX 32A) and 1 mL of the supernatant was diluted in 4 mL of 5% HNO3. However, this dilution led to the formation of precipitates. Thus, samples were then digested through the addition of 2.5 ml of 65% HNO3 (PanReac AppliChem, Germany, analytical grade) and 2.5 mL of 30% hydrogen peroxide (VWR Chemical, analytical grade) and heating at 70 °C during 1 h. Sample dilutions were prepared in 5% nitric acid. Afterward, a microwave plasma–atomic emission spectroscopy (MP-AES 4200, Agilent Technologies, USA) was used to determine the metals concentrations using standard calibration curves. Calibration curves were prepared from the following standard solutions in 5% nitric acid: Pd(NO3)2 in 0.5 M HNO3, Ce(NO3)3 in 5% HNO3, Al(NO3)3 in 0.5 M HNO3 and Fe(NO3)3 in 0.5 M HNO3, Nd2O3 in 2–3% HNO3, La2O3 in 2% HNO3 (from Merck Certipur, Germany), PtCl2 in 5% HCl (from Sigma-Aldrich standard for AAS, EUA), Rh in 10% HCl (from Acros organics, USA) in a dilution series of 0, 1, 5, 10, 20, 30, 40 and 50 mg/L for all elements. The correlation coefficients R2 of all calibration curves were above 0.99.
The bio-reduction of Pd2+ ions and particle formation was studied by UV–visible spectral analysis using wavelengths between 300 to 700 nm with 1 nm intervals in a BioTek Synergy 4 microplate reader (BioTek Instruments Inc., USA). The optical absorption spectra of the Pd2+ solution (100 mg/L) (Gaikwad and Rothenberg 2006), of a 5% (v/v) dilution of R. idaeus extract in 0.02 M HCl and of a 5% (v/v) dilution of R. idaeus extract in 100 mg/L of Pd2+ solution (1 h after reaction) (Sheny et al. 2012; Siddiqi and Husen 2016) were investigated.
Fourier transform infrared spectroscopy (FTIR) measurements of a Pd2+ solution, the R. idaeus extract and the mixture of Pd2+ solution and R. idaeus extract (Siddiqi and Husen 2016) were taken using a Nicolet iN10MX micro-FTIR (Thermo Scientific, USA) equipped with a MCT detector cooled with liquid nitrogen. Analyses were conducted in reflection mode, by spreading a drop of the sample onto a reflectance holder. Spectra were collected in the infrared region (from 4000 to 675 cm−1). Three measurements were taken for each sample to assure the robustness of the analysis.
The particles obtained from applying the R. idaeus extract to the H98 leachate were centrifuged and washed with 96% (v/v) ethanol for 1 h under orbital shaking and then centrifuged (2800 g for 60 min at room temperature) for liquid removal and finally dried under vacuum. (Two washing cycles were performed.) Subsequently, scanning electron microscopy–energy-dispersive X-ray spectroscopy (SEM–EDX) analysis were carried out using a 324 S3700N SEM system (Hitachi, Japan) coupled to a XFlash 5010 SDD EDS Detector (Bruker, Germany). The samples were analyzed at low vacuum (40 Pa) with an accelerating voltage of 5 and 20 kV. Then, the particles obtained from the prior washing step with 96% ethanol were further washed for two cycles with pure acetone to remove excess of organic compounds as follows: mixed during 1 h under orbital shaking and then centrifuged (2800 g for 60 min at room temperature) for liquid removal and dried under vacuum. Thereafter, Scanning Transmission Electron Microscope (STEM) imaging was carried out on a Titan ChemiSTEM (Thermo Fisher Scientific, Waltham, USA) microscope operating with a field emission gun and aberration corrector on the probe and four energy-dispersive X-ray spectroscopy (EDX) detectors operating at 200 kV. Before the STEM analysis, particles were resuspended in ethanol and sonicated for 30 min to pulverize the samples. Then, 5 μl of each sample were dispersed on copper coated grids, and the grids were dried and stored in a desiccator until imaging.
Results and discussion
Metals removal from unimetallic solutions
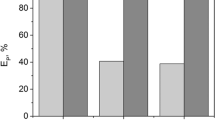
The experiments with the unimetallic solutions revealed similar metal removal trends with the three plant extracts (Fig. 1). Highest removals, significantly different from the controls, were achieved for Pd2+, varying from 48 to 81%. At a lower extent, Pt2+ and Fe3+ were also significantly removed in the three experiments: Pt2+ removals were between 19 and 33%, and Fe3+ removals were between 15 and 37%. On the other hand, the removals of Ce3+ and Al3+ were, in general, not significantly different from those achieved in the controls and were just in the range from 3 to 28%.
Metal removals achieved after 1 h and 48 h of mixing C. ladanifer, E. andevalensis and R. idaeus 70% ethanolic extracts at a 1/1 (v/v) ratio to the unimetallic solutions (~ 100 mg/L) at room temperature (25 ± 3 °C). Results for controls, consisting of the addition of just 70% ethanol (without plant extract), are included. Removals with the same letters do not significantly differ at 0.05 level (ANOVA and Tukey Kramer's tests)
It must be emphasized that the removals achieved in the unimetallic solutions of Pt2+, Pd2+, Al3+ and Ce3+ were not caused by alkalization, since the pH values in the mixtures were below the estimated pH at which these metals are expected to start precipitating according to simulations using the Medusa-Hydra software (Puigdomenech 2015) (Table Online Resource1). However, in the experiment with the unimetallic solution of Fe3+, the pH values in the mixtures were above the theoretical estimates at which this ion would start forming solid complexes of Fe(OH)2·7Cl0.3; therefore, in this case, the removals could have been caused by pH changes. In addition, generally reaction time of 1 h and 48 h does not have high impact on the removal of metals from the unimetallic solutions (Fig. 1), indicating the fast kinetics of the reactions between phytochemicals and studied metal ions.
Hence, the results indicate a tendency toward a higher interaction of plant compounds with Pd2+ than with the other tested metal ions, especially in the experiments with the extracts of R. idaeus and E. andevalensis (Fig. 1). This confirms the potential of some plant extracts for Pd2+ separation from solutions, which was already demonstrated using unimetallic solutions and several plant materials, as for example extracts of leaves from Euphorbia granulate (Nasrollahzadeh and Mohammad Sajadi 2016), from Soybean (Glycine max) (Kumar Petla et al. 2012), from Origanum vulgare (Seyedi et al. 2018), from Eryngium caeruleum (Saleh et al. 2021), or, for instance, extracts of peels from Punica granatum (Şahin Ün et al. 2020), and from orange (Wicaksono et al. 2020). In fact, this is a very interesting feature with potential applications for the recovery of this valuable metal from wastewaters and/or leachates of waste materials. Therefore, further experiments were performed using real solutions containing PGMs, aiming to assess the potential of the three plant extracts prepared in this work for the recovery of Pd from complex matrices.
PGM recovery from SACC leachates
SACC leachates characterization
The metal-bearing leachates from spent SACCs (I95 and H98) were diluted in a 1/6 ratio (v/v) in demineralized water before being used in the experiments with plant extracts and were analyzed in terms of the main metals, pH and chloride anion (Cl−) associated to the acidic matrices (Table 1). The leaching step provided satisfactory results for the PGMs usually present in these devices: Pt and Rh were leached from I95, while Pt and Pd were leached from H98. However, as expected, other metals which can be considered as contaminants in the recovery process were also leached (Table 1). Although the focus of this paper is not to study the efficiency of the leaching process, it is important to have realistic leaching liquors for target metal recovery studies.
Metals removal from SACCs leachates
In the experiments using the SACC leachates the pH values in the tests and in the controls were all below the values at which precipitation of the tested metals starts to occur, according to the theoretical simulations (Table Online Resource2). This suggests that none of the observed removals were caused by pH changes. In fact, the results were encouraging regarding the use of plant extracts for the recovery of metals from the ACCs leachates. The removals of the tested PGMs were significantly higher in the tests with plant extracts than in the controls and were in general higher than the removals of contaminant metals (Figs. 2 and 3). This relative specificity for the target metals was particularly evident in the experiments with E. andevalensis and R. idaeus extracts added to the H98 leachate, in which precipitations of 60% to 90% of the initial Pd2+ (~ 200 mg/L) and 60% to 75% of the initial Pt2+ (~ 45 mg/L) was achieved. Also, the differences observed in elements removals between the two studied reaction times (1 h. and 48 h.) were relatively small in both leachates when using each of the three plant extracts, as previously reported for the unimetallic solutions.
Removal of main metals present in the diluted (1/6 (v/v)) I95 leachate solution, achieved after 1 h and 48 h addition of C. ladanifer, E. andevalensis and R. idaeus 70% ethanolic extracts at a 1/1 (v/v) ratio at room temperature (25 ± 3 °C). Results for controls, consisting of the addition of just 70% ethanol (without plant extract), are included. Removals with the same letters do not significantly differ at 0.05 level (ANOVA and Tukey Kramer's tests)
Removal of main metals present in the diluted (1/6 (v/v)) H98 leachate solution, achieved after 1 h and 48 h addition of C. ladanifer, E. andevalensis and R. idaeus 70% ethanolic extracts at a 1/1 (v/v) ratio at room temperature (25 ± 3 °C). Results for controls, consisting of the addition of just 70% ethanol (without plant extract), are included. Removals with the same letters do not significantly differ at 0.05 level (ANOVA and Tukey Kramer's tests)
Despite the encouraging results, there was an incomplete separation of Pd and Pt in these experiments, which can possibly be explained by the increasing difficulty of new metal nuclei formation due to the lowering chance of metal atoms encounters as their concentrations decrease. Accordingly, the initial lower concentrations of PGMs in the I95 SACC leachate could be a possible explanation for the lower removals achieved with it. Thirumurugan et al. (2016) suggested operation processes at high temperatures and long reaction times for a complete recovery of Pd by plant extracts. However, since the aim of this study is to investigate the potential of plant extracts in PGM recovery in an eco-friendly and economically viable process suitable for industrial-scale operations, experiments at high temperatures were avoided.
The only metal contaminant with removal percentages significantly higher in the tests than in the controls was Fe. However, the removals were below ~ 40% and the initial concentration of iron was relatively low ([Fe] < 30 mg/L) in both diluted leachates I95 (1/6) and H98 (1/6), comparing with other contaminants (Table 1). Moreover, some removal of Ce was observed in the experiments, both in the tests and in the controls, and although the removal percentages were low (< 18%), they may correspond to high amounts of this metal due to its high initial concentrations (~ 671 and ~ 751 mg/l in diluted (1/6) leachates of I95 and H98). Despite this possible contamination of the PGM recovered by this strategy, they could still be useful, depending on their applications. It is reported that the combination of oxide and metal nanocomposites into a hybrid composite gives rise to new collective properties, which are different to the properties of each individual component (Tan et al. 2014). For instance, Wang et al. reported that the addition of CeO2 to Pd nanoparticles improves the catalytic activity (Wang et al. 2009). Moreover, Rajesh et al. (2019) and Tan et al. (2014) reported that the CeO2 content highly increases the electrochemical reaction of Pd/CeO2 and causes very efficient mass transport in ethanol and methanol oxidation reactions than Pd particles alone. Other examples of Pd/CeO2 nanocomposites applications are: as efficient formic acid electrooxidation catalyst (Feng et al. 2012) and high-performance catalytic electrodes for fuel cell applications (Kannan et al. 2015).
E. andevalensis only exists in nature in small areas near lagoons contaminated with acid mine water in the Iberian Peninsula, so it is not interesting as a raw material for hydrometallurgical processes. Despite this, it may be interesting to study the interactions of target metals with compounds present in the leaves of this shrub that grows in such extreme environments, because it may lead to the discovery of biomolecules with interesting properties that could inspire the production of new synthetic molecules. Moreover, if future studies reveal more potential effectiveness of the E. andevalensis extract for different industrial purposes with economical viabilities, new plantations of this plant as a new bio-economic enterprise can be hypothesized. On the contrary, R. idaeus leaves are available in large quantities due to the agro-industrial production of red raspberry fruits. For this reason, the further study on the PGM recovery mechanism and on the characterization of the obtained precipitates was focused on the extract from these leaves.
PGM recovery mechanism
The high percentages of precipitation achieved in the unimetallic solutions containing Pd2+ and Pt2+ by the addition of 70% ethanolic extracts of leaves from R. idaeus, and the particularly high and selective precipitations achieved for those metals in the HCl-based leachate from the H98 SACC with these extracts, might have been caused by two different phenomena: the reduction of the soluble ions of these metals into their metallic/elemental state and aggregation in nanoparticles, caused by the known high reducing properties of plant extracts, and/or the specific binding of these ions to some plant compounds resulting in their precipitation. Pd2+ and Pt2+ ions have higher reduction potential compared to other metals, as it can be seen in metals’ reduction potential tables published by Bratsch (1989) and Bard et al. (1985). Moreover, several works revealing the ability of different plants hydroalcoholic extracts to reduce and form Pd nanoparticles have been published (Khodadadi et al. 2017; Seyedi et al. 2018; Rostami-Vartooni et al. 2019).
UV–visible spectroscopy
The UV–visible spectra analysis is a helpful tool for the determination of the metal precursors' reduction (Zhang et al. 2007; Borodko et al. 2010). In this study, reduction of the Pd2+ ion was tracked by assessing the absorbance spectra in the optical range from 300 to 700 nm on the following samples: (i) a 100 mg/L Pd2+ solution in 0.02 M HCl, (ii) the R. idaeus extract added in a 5% (v/v) ratio to a 0.02 M HCl solution and (iii) the R. idaeus extract added in a 5% (v/v) ratio to the 100 mg/L Pd2+ solution in 0.02 M HCl (Fig. 4).
UV–visible spectrum of (red) 100 mg/L Pd2+ solution in 0.02 M HCl, (green) R. idaeus extract added in a 5% (v/v) ratio to a 0.02 M HCl solution (1 h after mixing) and (black) R. idaeus extract added in a 5% (v/v) ratio to the 100 mg/L Pd2+ solution in 0.02 M HCl (1 h after mixing at room temperature (25 ± 3 °C))
The initial Pd2+-bearing solution has high absorbance at 300 nm, which decays as the wavelength raises to ~ 350 nm, and then, one peak occurs at approximately 397 nm. With the plant extract the absorbance at 300 nm is even higher, probably due to the presence of plant compounds, but it also decays continuously as the wavelength raises and the peak at 397 nm does not exist. In the mixture with R. idaeus extract added to the Pd2+ solution, the previously observed peak at 397 nm in the Pd2+ solution disappears under a continuum absorption spectrum. Thus, this evolution of the absorption spectra could be due to a change of the Pd specimen and Pd nanoparticle formation and/or change of plant compounds and/or formation of complexes between Pd atoms and plant compounds. The peak at 397 nm has been attributed to the presence of Pd2+ ions and its disappearance under an increased continuum absorption spectra reported as an indication of Pd2+ ions reduction into Pd0 nanoparticles (Kumar Petla et al. 2012). The continuum absorption of UV–visible spectra in the solution containing Pd0 nanoparticles is due to the Surface Plasmon Resonance (SPR) of the Pd0 nanoparticles (Mulvaney 1996).
Fourier transform infrared spectroscopy
FTIR analysis was performed for the detection of biomolecules’ functional groups present in the R. idaeus hydroalcoholic extract that were possibly associated to bio-reduction of Pd2+ ions and stabilization of the formed Pd0 nanoparticles. Figure 5 presents the FTIR spectra of R. idaeus extract before and after reaction with Pd2+ ions.
FTIR spectra of (above) R. idaeus hydroalcoholic extract diluted for analysis to a 15% (v/v) ratio in 0.02 M HCl and (below) R. idaeus extract added in a 50% (v/v) ratio to a 100 mg/L Pd2+ solution in 0.02 M HCl and 1 h after mixing diluted for analysis to a 7.5% (v/v) ratio in 0.02 M HCl at room temperature (25 ± 3 °C)
The spectra of the R. idaeus extract before the reaction revealed several peaks in different regions, suggesting the complex nature of the biomolecules present in the extract: the peaks appearing at 1059, 1098 and 2989 cm−1 correspond to the ethanol (Silverstein and Webster 1997), which is the solvent of the plant extract, while the peaks observed at 841, 890, 1462, 1691, 1920, 2138, 2505 and 3578 cm−1 correspond to plant compounds. After the mixture of R. idaeus extract with Pd2+ solution, some peaks corresponding to functional groups of plant compounds were shifted to a higher wave number. The curve of the R. idaeus extract showed a peak at 841 cm−1, corresponding to the C–Cl stretching in the alkyl halides, that after the reaction with Pd2+ solution shifted to 848 cm−1. The peak at 890 cm−1 changed to 891 cm−1 and is attributed to the C=C bending in alkenes. A weak band observed at 1462 cm−1 shifted to 1463 cm−1, indicating the involvement of the C–H bending of the methylene group existing in the plant extract. Another peak at 1691 cm−1, related to (NH)C=O and/or C=O stretching vibrations groups of conjugated aldehydes and carboxylic acids, changed to 1694 cm−1, suggesting the participation of some phenolic acids or organic acids (Lu and Hsieh 2012) mainly as ellagic acids (Oszmiański et al. 2011) in the bio-reduction of Pd2+ ions. Moreover, three peaks at 1920, 2138 and 2505 cm−1, which correspond to C–H bending of the aromatic compounds, N=C=N stretching of the carbodiimide group and S–H stretching in the thiol group respectively, were vanished after the reaction of plant extract with Pd2+ solution. The C–H bending of the aromatic compounds observed at 1920 cm−1 could be related to the presence of high concentrations of phenolic compounds in the R. idaeus extract including flavonoids, tannins and anthocyanins as reported by Veljković et al. (2018). Moreover, the band observed at 2138 cm−1 is related to N=C=N stretching of the carbodiimide group and might be an indication of the amino acids or proteins (Fiehn et al. 2000) and/or anthocyanin (Demirbas et al. 2019; Ekrikaya et al. 2021). The peak observed at 2505 cm−1 of S–H stretching in the thiol group could be related to the cysteine-rich phytochemicals (Harada et al. 2002) and/or metallothioneins (Leszczyszyn et al. 2013), since both are known for their high metal interaction potential (Nobahar et al. 2021). The presence of high levels of cysteine in R. idaeus hydroalcoholic extract has been previously reported by Komisarenko et al. (2021). The band observed at 3578 cm−1 can be related to the –OH vibrations of phenols, carboxylic acids or alcohols and/or -NH vibrations of proteins, peptides and amines.
The presence of high contents of polyphenols (2.6% to 6.9% (w/w)) in the R. idaeus dried leaves, principally as ellagic acids, is described in the literature (Oszmiański et al. 2011; Moreno-Medina et al. 2018). The second compound with high abundancy in R. idaeus leaves are known to be flavonoids, ranging from 0.46 to 1.05% of the dried leaves (w/w) (Gudej 2003). Based on a study by Oszmiański et al. (2011), a major fraction of the phenolic content of R. idaeus leaves is reported as flavonoids, comprising about 11% of the leaf extract powder weight. In addition, some other phenolic compounds such as caffeic and chlorogenic acid (Durgo et al. 2012), p-coumaric, ferulic, protocatechuic, gentisic, caffeoyltartaric, feruloyl tartaric, p-coumaroyl-glucoside acids, p-hydroxybenzoic, vanillic acids (Brandely 2006), as well as terpenoids including mono- and sesquiterpenes, like terpinolene and triterpenes, squalene and cycloartenol, are reported in R. idaeus leaves (Kylli 2010; Committee on Herbal Medicinal Products (HMPC) 2012).
In a study similar to the one here reported, Nasrollahzadeh et al. (2015) used hydroalcoholic leaf extract of Hippophae rhamnoides Linn with an aqueous solution of PdCl2 and reported the synthesis of stable Pd0 nanoparticles, suggesting that OH functional groups, carbonyl groups, stretching aromatic rings and C–OH stretching vibrations present in the structure of phenols and flavonoids of the plant extract may have had the most important role in Pd2+ reduction and stabilization of the synthesized Pd0 nanoparticles. Some other reports have also described different types of plant compounds in the groups of phenols (Behnia et al. 2019) and polyphenols (Ho Kim and Nakano 2005; Kim et al. 2007; Morisada et al. 2011; Khan et al. 2017) with high tendency to bind specifically to PGMs.
Moreover, there are some known interaction mechanisms of phenolic compounds with metals; for instance, the high potential of free radical scavenging through phenols’ functional groups, as well as their electron or proton donation capacity, are substantial in their metal interaction capacities (Kaurinovic and Vastag 2019). Moreover, metal interaction properties of phenols are also explained by the presence of nucleophilic aromatic rings in conjunction with some functional groups such as carbonyl, carboxyl and hydroxyl groups (Kulbat 2016; Liu et al. 2018).
Characterization of the particles
Particles washed with ethanol
The precipitates resulting from the highest PGM recovery obtained by mixing the 70% ethanolic extract of R. idaeus with the H98 leachate were washed twice with 96% ethanol aiming the removal of organic residues. Thereafter, the elemental composition of the particles was examined using SEM–EDX mapping. This analysis revealed that apart from the O and C of the plant compounds, which are spread throughout the area, Pd appears to be alone in some particles and together with Cl and Na in other particles, while Ce seems to be always together with P and Al (Figure Online Resource1). These three types of particles were selected for point analysis to study in more detail their elemental composition (Figure Online Resource2). At spot 1, apart from O and C, the main estimated normalized atomic percentages were 0.22% for Pd, 6.45% for Na and 7.77% for Cl, while at spot 2 they were 2.27% for Pd, 1.66% for Na and 1.51% for Cl. Thus, the co-occurrence of Pd with Cl and Na in the two spots might be due to the grouping of NaCl and Pd0 particles in the same aggregate and not due to the presence of a compound formed by the three elements, since in spot 1 and 2, Cl and Na were found in approximately the same ratios, most possibly as NaCl, while Pd was in these two spots at different ratios with these elements. At spot 3, the point analysis revealed, apart from O and C, the following main atomic normalized abundances: 0.73% of Ce, 1.51% Na, 1.47% Al, 1.35% P and a minor quantity of La (0.14%), an element which usually occurs together with Ce that was present in the H98 leachate. Probably some biomolecules from the plant leaves have bonded to these metal ions or their metal salts, making stable compounds. Indeed, it is known that many plant compounds can form stable complexes with metal ions or metal complexes generating precipitates (Nobahar et al. 2021). For example, it has been reported that plant extracts can induce the formation of CeO2 (Rajeshkumar and Naik 2018), Al2O3 (Ghotekar 2019) and La2O3 (Dabhane et al. 2020). However, according to Singh et al. (2020), the CeO2 particle formation by plant extracts requires high temperatures, and therefore, Ce particles are most probably not formed as CeO2. Moreover, the co-occurrence of Ce, P and Al in large particles could indicate the presence of a mineral with the three elements or a complex structure of different minerals aggregated with plant compounds. The presence of these elements in the same particles opens the possibility of florencite–(La) ((La,Ce)Al3(PO4)2(OH)6) and/or florencite–(Ce) (CeAl3(PO4)2(OH)6). Yet, there are other possible explanations. The observed phenomena could be complex/chelate formation of those elements with P-rich phytochemicals, which then bind forming aggregates due to molecular interactions, or the presence of P could also indicate that Ce and Al appear as interspersed metal phosphate salts.
In these particles, C and O were found as the main constituents (60–70% C and 20–35% O of normalized atomic abundances) (Figure Online Resource2), indicating the presence of high contents of organic material in the particles. This could be explained by (i) the reactivity of some plant compounds with Pd ions, reducing them into Pd0 nanoparticles and finally binding to the particles and acting as stabilizing agents (Ghosh et al. 2021), (ii) interaction of plant compounds with the other metal ions present in the leachate and (iii) insolubility of a part of the plant compounds in the acidic conditions of the leachate. However, the existence of plant material as main constituent of the particles may be unfavorable for future applications. Therefore, after the washing steps with 96% ethanol the particles were subjected to further two cycles of washing with pure acetone followed by centrifugation.
Particles washed with ethanol and acetone
The STEM-EDX mapping of the final particles (Fig. 6) showed strong Pd signals coincident with the particles’ positions, while the signals of the remaining elements detected (Cl, Na, Pt, Ce, P, Al and Fe) were weak and spread throughout all the area. This shows that the washing steps with acetone efficiently solubilized a major part of the plant compounds, which led to the solubilization of contaminant particles, thus leaving the Pd0 particles better purified.
STEM image of Pd nanoparticles obtained by adding 70% ethanolic extract of R. idaeus to the H98 leachate and corresponding EDX elemental mapping of Pd, Cl, Na, Pt, Ce, P, Fe and Al. Precipitates washed twice with ethanol, then two times with acetone and dry before analysis. Strong signal for Pd matching the particles; strong signal from Cl scattered in all the area with precipitates; and relatively weak signals for other elements in the spaces between particles
The relative abundances of elemental composition determined by STEM-EDS spectra from the whole area of one image (Figure Online Resource3) confirmed the particle purity (Table 2). Approximately 30% of the normalized atomic abundances corresponds to Pd, while the other metals have normalized atomic abundances between 0.3% and 3.4. In addition, ~ 40% corresponds to O and ~ 15% to Cl, which may be from phytochemicals of the plant extract and/or the acidic matrix of the leachate. Si concentration was not analyzed during the leaching experiments (Paiva et al. 2022). However, the detection of this metal in the final precipitate indicates its leaching from the SACCs monoliths as suggested by Abo Atia et al. (2021) and subsequent precipitation by phytochemicals.
The STEM images revealed particles with sizes below 20 nm, thus confirming the recovery of Pd as nanoparticles. The synthesized Pd nanoparticles were then analyzed with high-resolution scanning transmission microscopy (HRSTEM) for further characterization on the atomic scale. HRSTEM analysis of the particles showed very tiny Pd nanoparticles well dispersed with no aggregate formation. HRSTEM images revealed that the morphology of the nano-sized particles is generally quasi-spherical (Fig. 7A, B and D). Sizes of a total of 265 particles were automatically measured in HRSTEM images by ParticleSizer version 1.0.9 plug-in (Wagner and Eglinger 2021) on the Fiji-ImageJ software (Schindelin et al. 2012), revealing particles varying from 2.5 to 17 nm, with a Feret size average of 6 ± 2 nm, and fitting a normal distribution (for a 1% probability (α = 0.01) in a Kolmogorov–Smirnov test) (Fig. 7C). The presence of well stabilized and dispersed tiny nanoparticles could be due to the effectiveness of phytochemicals present in R. idaeus extract to act as capping and stabilizing agents. Finally, some HRSTEM images showed well defined adjacent lattice fringes on the particles (Fig. 7D) and the fast Fourier transform (FFT) pattern of a selected particle revealed diffraction spots indicating an interplanar lattice distance of ~ 0.22 nm (Fig. 7E), which is characteristic of (111) planes (Cheong et al. 2010; Sarıbıyık et al. 2020). In addition, the selected area electron diffraction (SAED) pattern of the same particle (Fig. 7F) confirmed the crystalline nature of the nanoparticles. Thus, this work confirms the potential application of R. idaeus extracts for valuable metal nanoparticle synthesis with high stability, as previously reported by Singh et al. (2020) for silver and by Demirbas et al. (2019).
A, B TEM images of the Pd nanoparticles obtained by adding 70% ethanolic extract of R. idaeus to the H98 leachate C histogram representing the size distribution of Pd nanoparticles, D magnified high-resolution TEM with lattice fringes visible on the particles, E fast Fourier transform (FFT) pattern of a single nanoparticle (red square in figure D) and F) selected area electron diffraction (SAED) patterns of the same nanoparticle, with visible spectra profile rings. Precipitates washed twice with ethanol, then two times with acetone and were dried before analysis
Economic and environmental perspectives
Concerning the economic and environmental aspects, it is crucial to understand the balance between material, energy flow and waste generation (Kliestik et al. 2020; Maroušek and Trakal 2022). Hydrometallurgical processes using HCl–H2O2-based leaching solvents at moderate acidity (~ 3 M to 6 M HCl and 1% to 10% H2O2) and with high PGM recovery rates, are considered Mild and Green systems economically favorable since no hazardous gas or by-products are generated and low-temperature reactions are used (Karim and Ting 2021; Yakoumis et al. 2021). For example Hereaus, Germany, which is one of the PGM refining corporations from SACCs in the Europe, employs hydrometallurgical processes including HCl–H2O2 leaching process (Jha et al. 2013; Padamata et al. 2020). The leachates used in this work (HCl = 11.6 M, H2O2 = 1% (w/v)) were obtained in harsh medium. Nevertheless, they were diluted in water at a ratio of 1:6 to achieve milder conditions before the recovery of metals with plant extracts. Like this, it was shown that plant-based extracts can be used to recover metals from milder leaching solutions, and it was also shown that even when more aggressive solutions are used in the leaching step, plant-based extracts can be used to recover metals if a previous dilution is performed to lower the acidity.
Regarding the PGM separation from the leaching solution, different methods that are described in the literature can be employed, such as reduction, chemical precipitation and solvent extraction (Saguru et al. 2018). Some of these methods are described for their high recovery efficiency, however, there is always space for improvements in economic and environmental aspects (Karim and Ting 2021). For example, PGM solvent extraction results obtained with model solutions are usually more promising than the application of the solvents to real leaches, and PGM solvent extraction reports with real SACCs leachates are generally still missing in recent literature (Paiva et al. 2022). Moreover, solvent extraction makes use of organic extractants (e.g., Aliquat 336 and Cyanex 301) and solvents to dilute them (e.g., kerosene) which have a cost, and after a certain time of use require waste management (Yakoumis et al. 2021). Both leachates used in this work, I95 and H98 leachates, have already been used for solvent extraction experiments by several commercial extractants and the results showed that the most promising were Cyanex® 471X and Cyphos® IL 101, with extraction efficiencies over 99%. However, in both cases there was a high (> 99%) co-extraction of iron, which could not be totally removed from the extractant before Pd stripping, and the final stripping efficiencies of Pd were low (< 50%) (Paiva et al. 2022). The method proposed in this study uses an ethanolic extract of R. idaeus leaves, which are agricultural wastes, for the direct separation of Pd from the leaching solution as nanoparticles. Thus, it eliminates the need to purchase chemicals for direct precipitation and/or extractants and their solvents for a previous separation step. Table 3, summarizes the approximate operational costs of the proposed method regarding the PGM leaching and recovery from SACCs.
In order to evaluate the financial viability of process it is necessary to predict the process costs per weight of recovered product. The average Pd content in ACCs is considered as ~ 1580 mg per ACC and the average weight of honeycombs in each ACC is about 0.737 kg (Yakoumis et al. 2018). The leaching step proposed in this study allows a recovery efficiency of ~ 99% (Paiva et al. 2022); thus, ~ 2.14 g of Pd can be leached per Kg of ACC combs. Afterward, ~ 1.93 of Pd nanoparticles can be recovered from that leachate using an extract of R. idaeus leaves, according to the ~ 90% separation efficiency achieved in our work. Therefore, the estimated operation cost (Table 3) expressed per weight of recovered product is 20.36 € (or 19.87 $ on 23 Sept. 2022) / g of Pd particles. It must be said that this cost estimate is based on non-negotiated prices and relatively small quantities. It is likely possible to lower costs with more thorough market research and negotiations for bulk orders. Plus, to reduce the costs, most probably the leaves obtained for free as a waste can be air-dried without heat before grinding for extract preparation.
In this method, not only Pd is separated from the solution, but Pd nanoparticles are produced, which rises the financial viability of the process. For example currently, the price of metallic Pd in the stock market is about 2000 $ per ounce (~ 65 $ per gram) (GoldPriceOZ 2022), while the price per gram of highly pure (> 99.5%) Pd nanoparticles with sizes < 1 μm is 191 $ (Sigma-Aldrich 2022a) and < 25 nm is 1942 $ (Sigma-Aldrich 2022b). Even though the produced Pd nanoparticles in this study have much less purity, ~ 75% excluding oxygen (Table 2), they have still various application potentials due to their very small size (2.5–17 nm) and large surface area. Therefore, the price of the synthesized nanoparticles can be significantly higher than metallic Pd, enhancing the financial viability of the process. Indeed, there are a wide range of applications known for Pd nanoparticles that may require different levels of purity, as for example: photocatalytic activity of phenol red dye (Kora and Rastogi 2018), removal of pathogenic microbes from wastewaters (Mishra et al. 2019), catalytic reduction of 4-nitrophenol (Tuo et al. 2017), decomposition of methyl orange (Ahmed et al. 2018) to bio-sensing, fuel cells and other fields (Mubeen et al. 2007; Wang et al. 2012; Azharuddin et al. 2019).
Finally, it can be said that to enhance the financial feasibility of the process there is still room to optimize the particle washing steps and reach purities greater than 99.5%, as well as to recover other PGMs (Pt and Rh) or even other metals with market value (Ce, La, Nd) that may be preset in the leachate by a previous or subsequent application of other methods such as molecular recognition technology (Zheng et al. 2021) or ion exchange and chelating resins (Lee et al. 2020).
Conclusions
The current study reinforces the idea that plant extracts include a variety of compounds with different metal binding potentials and that their interactions through different mechanisms results in metal precipitation. It was found that 70% ethanolic extracts from leaves of R. idaeus can induce high precipitation of Pd2+ and Pt2+ ions from aqueous solutions, thus suggesting potential use of polar extracts for the recovery of these metals from different metal-bearing waters. More specifically, it was demonstrated that the addition of such extracts from R. idaeus leaves to SACC leachates allows to recover ~ 90% of Pd by inducing the production of a precipitate containing agglomerates of particles (of sizes up to 10 µm) having Pd and other elements such as Na, Cl, Ce, P and Al, along with plant material, which after washing with acetone results in a more purified (~ 75%) precipitate with Pd0 nanoparticles (6 ± 2 nm) and traces of those other elements present in the spaces between particles.
The method has a rough cost estimate of ~ 20 $ per gram of Pd particles (excluding manpower). However, this estimate was calculated with laboratory-scale operations and most probably can be improved in a large-scale process.
R. idaeus is an agricultural crop widely cultivated in Europe, Asia and North America for its fruits, and its leaves are the main waste of the fruits’ harvesting and therefore are available in large quantities. The method developed in this study using ethanolic extracts of these wastes could be applied for Pd0 nanoparticle synthesis from both unimetallic Pd2+ solutions or multimetallic leachates. In the first case, such green synthesis would produce pure Pd0 nanoparticles without other metals as contaminants. In the second case, the produced Pd0 nanoparticles would have a certain level of contamination with other metals, depending on the composition of the used leachate.
Regarding the Pd recovery mechanism, after taking all the studies of this work into account, it can be suggested that the involved mechanism in Pd recovery from the solution as nanoparticles was neither adsorption or precipitation, since in both cases, Pd nanoparticle formation is not possible and all Pd recovered from the solution with phytochemicals would have been washed out along with phytochemicals from the precipitates during washing steps with ethanol and acetone. Thus, the most probable mechanism involved in Pd recovery is reduction, since results in the UV–visible spectroscopy revealed signs of Pd2+ reduction and Pd nanoparticle formation. Moreover, FTIR analysis revealed changes after mixing the Pd2+ solution with plant extract in different functional groups (such as OH group) that are mainly related to phenols and polyphenols and flavonoids, which are known as compounds with high capacity to reduce PGMs.
For the improvement of the technology aiming a highly sustainable circular economy more investigations are required, including: improvement of phytochemical extraction by more available and cheaper extractants, mainly as water; combining other recycling methods to recover Pt and Rh.; optimizing the reaction conditions to achieve higher recovery efficiencies; optimization of the metallic particle washing steps to minimize the amount of solvents used and to improve the particle purity, thus drastically raising the market value of the particles; identification of the main bioactive compounds interacting with Pd followed by their purification from the extract; and applying the proposed process to other ACC leaching solutions such as those from bio-leaching.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available because the manuscript has the data included in figures, tables and electronic supplementary material and there is not additional data type that justifies availability. Nevertheless, any further information will be available from the corresponding author on reasonable request.
References
Abo Atia T, Wouters W, Monforte G, Spooren J (2021) Microwave chloride leaching of valuable elements from spent automotive catalysts: Understanding the role of hydrogen peroxide. Resour Conserv Recycl 166:105349. https://doi.org/10.1016/j.resconrec.2020.105349
Abreu MM, Tavares MT, Batista MJ (2008) Potential use of Erica andevalensis and Erica australis in phytoremediation of sulphide mine environments: São Domingos, Portugal. J Geochem Explor 96:210–222. https://doi.org/10.1016/j.gexplo.2007.04.007
Abreu MM, Santos E, Fernandes E, Batista MJ, Ferreira M (2011) Accumulation and translocation of trace elements in Cistus ladanifer L. from IPB Portuguese mining areas. Rev Ciências Agrárias (Portugal) 34(2):44–56
Ahmed E, Kalathil S, Shi L et al (2018) Synthesis of ultra-small platinum, palladium and gold nanoparticles by Shewanella loihica PV-4 electrochemically active biofilms and their enhanced catalytic activities. J Saudi Chem Soc 22:919–929. https://doi.org/10.1016/j.jscs.2018.02.002
Azharuddin M, Zhu GH, Das D et al (2019) A repertoire of biomedical applications of noble metal nanoparticles. Chem Commun 55:6964–6996. https://doi.org/10.1039/C9CC01741K
Baghalha M, Khosravian Gh H, Mortaheb HR (2009) Kinetics of platinum extraction from spent reforming catalysts in aqua-regia solutions. Hydrometallurgy 95:247–253. https://doi.org/10.1016/j.hydromet.2008.06.003
Bahaloo-Horeh N, Mousavi SM (2022) A novel green strategy for biorecovery of valuable elements along with enrichment of rare earth elements from activated spent automotive catalysts using fungal metabolites. J Hazard Mater 430:128509. https://doi.org/10.1016/j.jhazmat.2022.128509
Bard AJ, Parsons R, Jordan J (1985) International Union of pure and applied chemistry. N Y M Dekker
Behnia A, Fard MA, Boyle PD, Puddephatt RJ (2019) Complexes containing a phenol-platinum(II) hydrogen bond: synthons for supramolecular self-assembly and precursors for hydridoplatinum(IV) complexes: complexes containing a phenol-platinum(II) hydrogen bond: synthons for supramolecular self-assembly and precursors for hydridoplatinum(IV) complexes. Eur J Inorg Chem 2019:2899–2906. https://doi.org/10.1002/ejic.201900480
Benson M, Bennett CR, Harry JE et al (2000) The recovery mechanism of platinum group metals from catalytic converters in spent automotive exhaust systems. Resour Conserv Recycl 31:1–7. https://doi.org/10.1016/S0921-3449(00)00062-8
Borodko Y, Lee HS, Joo SH et al (2010) Spectroscopic study of the thermal degradation of PVP-capped Rh and Pt nanoparticles in H2 and O2 environments. J Phys Chem C 114:1117–1126. https://doi.org/10.1021/jp909008z
Brandely P (2006) Raspberry leaf. In British herbal compendium. A handbook of scientific information on widely used plant drugs. British Herbal Medicine Association, Bournemouth
Bratsch SG (1989) Standard electrode potentials and temperature coefficients in water at 298.15 K. J Phys Chem Ref Data 18:1–21. https://doi.org/10.1063/1.555839
Cabezudo B, Rivera J (1980) Notas taxonómicas y corológicas sobre la Flora de Andalucía occidental. 2: Erica andevalensis Cabezudo y Rivera sp. nov. Lagascalia 9:223–226
Cheong S, Watt JD, Tilley RD (2010) Shape control of platinum and palladium nanoparticles for catalysis. Nanoscale 2:2045. https://doi.org/10.1039/c0nr00276c
Committee on Herbal Medicinal Products (HMPC) (2012) Assessment Report on Rubus idaeus L., Folium.
Costa MC, Assunção A, da Costa AMR et al (2013) Liquid-liquid extraction of platinum from chloride media by dimethyl-dicyclohexyltetradecylmalonamide. Solvent Extr Ion Exch 31:12–23. https://doi.org/10.1080/07366299.2012.700588
Dabhane H, Ghotekar S, Tambade P, Medhane V (2020) Plant mediated green synthesis of lanthanum oxide (La2O3) nanoparticles: a review. Asian J Nanosci Mater
Demirbas A, Büyükbezirci K, Celik C et al (2019) Synthesis of long-term stable gold nanoparticles benefiting from red raspberry (Rubus idaeus ), strawberry ( Fragaria ananassa ), and blackberry (Rubus fruticosus ) extracts-gold ion complexation and investigation of reaction conditions. ACS Omega 4:18637–18644. https://doi.org/10.1021/acsomega.9b02469
Durgo K, Belščak-Cvitanović A, Stančić A et al (2012) The bioactive potential of red raspberry (Rubus idaeus L.) leaves in exhibiting cytotoxic and cytoprotective activity on human laryngeal carcinoma and colon adenocarcinoma. J Med Food 15:258–268. https://doi.org/10.1089/jmf.2011.0087
Ekrikaya S, Yilmaz E, Celik C et al (2021) Investigation of ellagic acid rich-berry extracts directed silver nanoparticles synthesis and their antimicrobial properties with potential mechanisms towards Enterococcus faecalis and Candida albicans. J Biotechnol 341:155–162. https://doi.org/10.1016/j.jbiotec.2021.09.020
Feng L, Yang J, Hu Y et al (2012) Electrocatalytic properties of PdCeOx/C anodic catalyst for formic acid electrooxidation. Int J Hydrog Energy 37:4812–4818. https://doi.org/10.1016/j.ijhydene.2011.12.114
Fiehn O, Kopka J, Trethewey RN, Willmitzer L (2000) Identification of uncommon plant metabolites based on calculation of elemental compositions using gas chromatography and quadrupole mass spectrometry. Anal Chem 72:3573–3580. https://doi.org/10.1021/ac991142i
Fornalczyk A, Saternus M (2011) Catalytic converters as a source of platinum. Metalurgija 50(4):261–264
Gaikwad AV, Rothenberg G (2006) In-situ UV-visible study of Pd nanocluster formation in solution. Phys Chem Chem Phys 8:3669. https://doi.org/10.1039/b604665g
Ghosh A, Hegde RV, Gholap SS et al (2021) Green pathways for palladium nanoparticle synthesis: application and future perspectives. In: Hussain CM, Shukla SK, Mangla B (eds) Functionalized nanomaterials for catalytic application, 1st edn. Wiley, Hoboken, pp 303–328
Ghotekar S (2019) Plant extract mediated biosynthesis of Al2O3 nanoparticles- a review on plant parts involved, characterization and applications. Nanochemistry Res 4(2):163–169
GoldPriceOZ (2022) Palladium Price per Gram. https://www.goldpriceoz.com/palladium/palladium-price-per-gram/?utm_content=cmp-true. Accessed 20 Sep 2022
Granados-Fernández R, Montiel MA, Díaz-Abad S et al (2021) Platinum recovery techniques for a circular economy. Catalysts 11:937. https://doi.org/10.3390/catal11080937
Gudej J (2003) Kaempferol and quercetin glycosides from Rubus idaeus L. leaves. Acta Pol Pharm 60:313–316
Harada E, Yamaguchi Y, Koizumi N, Hiroshi S (2002) Cadmium stress induces production of thiol compounds and transcripts for enzymes involved in sulfur assimilation pathways in Arabidopsis. J Plant Physiol 159:445–448. https://doi.org/10.1078/0176-1617-00733
Hegde RV, Ghosh A, Jadhav AH et al (2021) Biogenic synthesis of Pd-nanoparticles using Areca Nut Husk Extract: a greener approach to access α-keto imides and stilbenes. New J Chem 45:16213–16222. https://doi.org/10.1039/D1NJ02858H
Ho Kim Y, Nakano Y (2005) Adsorption mechanism of palladium by redox within condensed-tannin gel. Water Res 39:1324–1330. https://doi.org/10.1016/j.watres.2004.12.036
Hu H, Wang F, Ding X et al (2022) Green fabrication of Pt nanoparticles via tea-polyphenols for hydrogen peroxide detection. Colloids Surf Physicochem Eng Asp 637:128201. https://doi.org/10.1016/j.colsurfa.2021.128201
Ilyas S, Srivastava RR, Kim H (2022) Mobilization of platinum and palladium from exhausted catalytic converters using bio-cyanide and an ionic-liquid as mass transport carriers. Green Chem 24:5204–5218. https://doi.org/10.1039/D2GC00874B
Ishak NAI, Kamarudin SK, Timmiati SN (2019) Green synthesis of metal and metal oxide nanoparticles via plant extracts: an overview. Mater Res Express 6:112004. https://doi.org/10.1088/2053-1591/ab4458
Ismail E, Kenfouch M, Dhlamini M et al (2017) Green biosynthesis of rhodium nanoparticles via Aspalathus linearis natural extract. J Nanomater Mol Nanotechnol. https://doi.org/10.4172/2324-8777.1000212
Izatt RM, Izatt SR, Izatt NE et al (2015) Industrial applications of molecular recognition technology to separations of platinum group metals and selective removal of metal impurities from process streams. Green Chem 17:2236–2245. https://doi.org/10.1039/C4GC02188F
Jafarifar D, Daryanavard MR, Sheibani S (2005) Ultra fast microwave-assisted leaching for recovery of platinum from spent catalyst. Hydrometallurgy 78:166–171. https://doi.org/10.1016/j.hydromet.2005.02.006
Jeon S, Tabelin CB, Park I et al (2020) Ammonium thiosulfate extraction of gold from printed circuit boards (PCBs) of end-of-life mobile phones and its recovery from pregnant leach solution by cementation. Hydrometallurgy 191:105214. https://doi.org/10.1016/j.hydromet.2019.105214
Jha MK, Lee J, Kim M et al (2013) Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: a review. Hydrometallurgy 133:23–32. https://doi.org/10.1016/j.hydromet.2012.11.012
Jimenez de Aberasturi D, Pinedo R, Ruiz de Larramendi I et al (2011) Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic converters. Miner Eng 24:505–513. https://doi.org/10.1016/j.mineng.2010.12.009
Kannan R, Jang H-R, Yoo E-S et al (2015) Facile green synthesis of palladium quantum dots@carbon on mixed valence cerium oxide/graphene hybrid nanostructured bifunctional catalyst for electrocatalysis of alcohol and water. RSC Adv 5:35993–36000. https://doi.org/10.1039/C5RA04226G
Karim S, Ting Y-P (2021) Recycling pathways for platinum group metals from spent automotive catalyst: a review on conventional approaches and bio-processes. Resour Conserv Recycl 170:105588. https://doi.org/10.1016/j.resconrec.2021.105588
Karim S, Ting Y-P (2022) Bioleaching of platinum, palladium, and rhodium from spent automotive catalyst using bacterial cyanogenesis. Bioresour Technol Rep 18:101069. https://doi.org/10.1016/j.biteb.2022.101069
Kaurinovic B, Vastag D (2019) Flavonoids and phenolic acids as potential natural antioxidants. In: Shalaby E (ed) Antioxidants. IntechOpen, London
Khan M, Albalawi GH, Shaik MR et al (2017) Miswak mediated green synthesized palladium nanoparticles as effective catalysts for the Suzuki coupling reactions in aqueous media. J Saudi Chem Soc 21:450–457. https://doi.org/10.1016/j.jscs.2016.03.008
Khodadadi B, Bordbar M, Nasrollahzadeh M (2017) Green synthesis of Pd nanoparticles at Apricot kernel shell substrate using Salvia hydrangea extract: catalytic activity for reduction of organic dyes. J Colloid Interface Sci 490:1–10. https://doi.org/10.1016/j.jcis.2016.11.032
Kim Y-H, Ogata T, Nakano Y (2007) Kinetic analysis of palladium(II) adsorption process on condensed-tannin gel based on redox reaction models. Water Res 41:3043–3050. https://doi.org/10.1016/j.watres.2007.02.016
Kliestik T, Nica E, Musa H, Poliak M, Mihai EA (2020) Networked, smart, and responsive devices in industry 4.0 manufacturing systems. Econ Manag Financ Markets 15(3):23–29
Komisarenko MA, Polischuk IM, Upyr TV, Saidov NB (2021) Study of Amino acid composition and immunomodulatory activity of Rubus idaeus alcoholic extract. Res J Pharm Technol 14:1329–1332. https://doi.org/10.5958/0974-360X.2021.00236.5
Kora AJ, Rastogi L (2018) Green synthesis of palladium nanoparticles using gum ghatti (Anogeissus latifolia) and its application as an antioxidant and catalyst. Arab J Chem 11:1097–1106. https://doi.org/10.1016/j.arabjc.2015.06.024
Kulbat K (2016) The role of phenolic compounds in plant resistance. Biotechnol Food Sci 80(2):97–108
Kumar Petla R, Vivekanandhan S, Misra M et al (2012) Soybean (Glycine Max) leaf extract based green synthesis of palladium nanoparticles. J Biomater Nanobiotechnology 03:14–19. https://doi.org/10.4236/jbnb.2012.31003
Kumari R, Samadder SR (2022) A critical review of the pre-processing and metals recovery methods from e-wastes. J Environ Manage 320:115887. https://doi.org/10.1016/j.jenvman.2022.115887
Kylli P (2010) Berry phenolics: isolation, analysis, identification, and antioxidant properties. Doctoral dissertation, Helsingin yliopisto
Kyriakakis G (2005) Extraction of gold from platinum group metal (PGM) ores. In: Developments in Mineral Processing. Elsevier, pp 897–917. https://doi.org/10.1016/S0167-4528(05)15036-4
LabAlley (2022a) Ethanol, 190 proof (95%). https://www.laballey.com/products/ethanol-190-proof-95-denatured-alcohol-histological?variant=36012350701723. Accessed 20 Sep 2022
LabAlley (2022b) Acetone 100% Lab Grade. https://www.laballey.com/products/acetone-lab?variant=40856192024731. Accessed 20 Sep 2022
Lanaridi O, Schnürch M, Limbeck A, Schröder K (2022) Liquid- and solid-based separations employing ionic liquids for the recovery of platinum group metals typically encountered in catalytic converters: a review. ChemSusChem. https://doi.org/10.1002/cssc.202102262
Lee J, Kurniawan HH-J et al (2020) Separation of platinum, palladium and rhodium from aqueous solutions using ion exchange resin: A review. Sep Purif Technol 246:116896. https://doi.org/10.1016/j.seppur.2020.116896
Leszczyszyn OI, Imam HT, Blindauer CA (2013) Diversity and distribution of plant metallothioneins: a review of structure, properties and functions. Metallomics 5:1146. https://doi.org/10.1039/c3mt00072a
Limjuco LA, Burnea FK (2022) Evaluation of dithiadiamide-based molecular ion imprinted polymer (MIIP) for selective recovery of platinum from acid-digested spent automobile catalytic converter (ACC) solution. MRS Commun 12:175–182. https://doi.org/10.1557/s43579-022-00158-9
Liu Y-S, Chang Y-C, Chen H-H (2018) Silver nanoparticle biosynthesis by using phenolic acids in rice husk extract as reducing agents and dispersants. J Food Drug Anal 26:649–656. https://doi.org/10.1016/j.jfda.2017.07.005
Lu P, Hsieh Y-L (2012) Cellulose isolation and core–shell nanostructures of cellulose nanocrystals from chardonnay grape skins. Carbohydr Polym 87:2546–2553. https://doi.org/10.1016/j.carbpol.2011.11.023
Ma Y, Oliveira RS, Freitas H, Zhang C (2016) Biochemical and molecular mechanisms of plant-microbe-metal interactions: relevance for phytoremediation. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00918
Maroušek J, Trakal L (2022) Techno-economic analysis reveals the untapped potential of wood biochar. Chemosphere 291:133000. https://doi.org/10.1016/j.chemosphere.2021.133000
Mishra V, Arya A, Chundawat TS (2019) High catalytic activity of Pd nanoparticles synthesized from green alga chlorella vulgaris in buchwald-hartwig synthesis of N-Aryl piperazines. Curr Organocatal 7:23–33. https://doi.org/10.2174/2213337206666190515091945
Monaci F, Leidi EO, Mingorance MD et al (2011) Selective uptake of major and trace elements in Erica andevalensis, an endemic species to extreme habitats in the Iberian Pyrite Belt. J Environ Sci 23:444–452. https://doi.org/10.1016/S1001-0742(10)60429-9
Moreno-Medina BL, Casierra-Posada F, Cutler J (2018) Phytochemical composition and potential use of Rubus species. Gesunde Pflanz 70:65–74. https://doi.org/10.1007/s10343-018-0416-1
Morisada S, Kim Y-H, Ogata T et al (2011) Improved adsorption behaviors of amine-modified tannin gel for palladium and platinum ions in acidic chloride solutions. Ind Eng Chem Res 50:1875–1880. https://doi.org/10.1021/ie102193a
Mubeen S, Zhang T, Yoo B et al (2007) Palladium nanoparticles decorated single-walled carbon nanotube hydrogen sensor. J Phys Chem C 111:6321–6327. https://doi.org/10.1021/jp067716m
Mulvaney P (1996) Surface plasmon spectroscopy of nanosized metal particles. Langmuir 12:788–800. https://doi.org/10.1021/la9502711
Nasrollahzadeh M, Mohammad Sajadi S (2016) Pd nanoparticles synthesized in situ with the use of Euphorbia granulate leaf extract: catalytic properties of the resulting particles. J Colloid Interface Sci 462:243–251. https://doi.org/10.1016/j.jcis.2015.09.065
Nasrollahzadeh M, Sajadi SM, Maham M (2015) Green synthesis of palladium nanoparticles using Hippophae rhamnoides Linn leaf extract and their catalytic activity for the Suzuki-Miyaura coupling in water. J Mol Catal Chem 396:297–303. https://doi.org/10.1016/j.molcata.2014.10.019
Nobahar A, Carlier JD, Miguel MG, Costa MC (2021) A review of plant metabolites with metal interaction capacity: a green approach for industrial applications. Biometals. https://doi.org/10.1007/s10534-021-00315-y
Oszmiański J, Wojdylo A, Gorzelany J, Kapusta I (2011) Identification and characterization of low molecular weight polyphenols in berry leaf extracts by HPLC-DAD and LC-ESI/MS. J Agric Food Chem 59:12830–12835. https://doi.org/10.1021/jf203052j
Padamata SK, Yasinskiy AS, Polyakov PV et al (2020) Recovery of noble metals from spent catalysts: a review. Metall Mater Trans B 51:2413–2435. https://doi.org/10.1007/s11663-020-01913-w
Paiva AP, Piedras FV, Rodrigues PG, Nogueira CA (2022) Hydrometallurgical recovery of platinum-group metals from spent auto-catalysts – Focus on leaching and solvent extraction. Sep Purif Technol 286:120474. https://doi.org/10.1016/j.seppur.2022.120474
Pantelidis G, Vasilakakis M, Manganaris G, Diamantidis G (2007) Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem 102:777–783. https://doi.org/10.1016/j.foodchem.2006.06.021
PGM Market Report (2018) Summary of platinum supply and demand in 2017, 2018. URL. Johns Matthey
Platinum, 2008 (2008) Platinum 2008, [WWW Document]
Puigdomenech I (2015) Hydra/medusa chemical equilibrium database and plotting software (database update: 01–01–2015; Hydra: 32 bit version 18 Aug. 2009; Medusa: 32 bit version 16 Dec. 2010). KTH R Inst Technol Stock
Rajesh D, Mahendiran C, Suresh C et al (2019) Hydrothermal synthesis of three dimensional reduced graphene oxide-multiwalled carbon nanotube hybrids anchored with palladium-cerium oxide nanoparticles for alcohol oxidation reaction. Int J Hydrog Energy 44:4962–4973. https://doi.org/10.1016/j.ijhydene.2019.01.025
Rajeshkumar S, Naik P (2018) Synthesis and biomedical applications of Cerium oxide nanoparticles – a review. Biotechnol Rep 17:1–5. https://doi.org/10.1016/j.btre.2017.11.008
ReAgent (2022a) Hydrochloric acid. In: Chemicals. https://www.chemicals.co.uk/hydrochloric-acid. Accessed 20 Sep 2022
ReAgent (2022b) Hydrogen peroxide 30%. In: Chemicals. https://www.chemicals.co.uk/hydrogen-peroxide-tech. Accessed 20 Sep 2022
Rossini-Oliva S, Abreu MM, Leidi EO (2018) A review of hazardous elements tolerance in a metallophyte model species: Erica andevalensis. Geoderma 319:43–51. https://doi.org/10.1016/j.geoderma.2017.12.035
Rostami-Vartooni A, Rostami L, Bagherzadeh M (2019) Green synthesis of Fe3O4/bentonite-supported Ag and Pd nanoparticles and investigation of their catalytic activities for the reduction of azo dyes. J Mater Sci Mater Electron 30:21377–21387. https://doi.org/10.1007/s10854-019-02514-3
Saguru C, Ndlovu S, Moropeng D (2018) A review of recent studies into hydrometallurgical methods for recovering PGMs from used catalytic converters. Hydrometallurgy 182:44–56. https://doi.org/10.1016/j.hydromet.2018.10.012
Saleh EAM, Khan AU, Tahir K et al (2021) Phytoassisted synthesis and characterization of palladium nanoparticles (PdNPs); with enhanced antibacterial, antioxidant and hemolytic activities. Photodiagnosis Photodyn Ther 36:102542. https://doi.org/10.1016/j.pdpdt.2021.102542
Santos ES, Abreu MM, Nabais C, Magalhães MCF (2012) Trace element distribution in soils developed on gossan mine wastes and Cistus ladanifer L. tolerance and bioaccumulation. J Geochem Explor 123:45–51. https://doi.org/10.1016/j.gexplo.2012.05.006
Santos ES, Abreu MM, Batista MJ et al (2014) Inter-population variation on the accumulation and translocation of potentially harmful chemical elements in Cistus ladanifer L. from Brancanes, Caveira, Chança, Lousal, Neves Corvo and São Domingos mines in the Portuguese Iberian Pyrite Belt. J Soils Sediments 14:758–772. https://doi.org/10.1007/s11368-014-0852-1
Santos ES, Abreu MM, Magalhães MCF (2016) Cistus ladanifer phytostabilizing soils contaminated with non-essential chemical elements. Ecol Eng 94:107–116. https://doi.org/10.1016/j.ecoleng.2016.05.072
Sarıbıyık OY, Weilach C, Serin S, Rupprechter G (2020) The effect of shape-controlled Pt and Pd nanoparticles on selective catalytic hydrodechlorination of trichloroethylene. Catalysts 10:1314. https://doi.org/10.3390/catal10111314
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Seyedi N, Saidi K, Sheibani H (2018) Green synthesis of pd nanoparticles supported on magnetic graphene oxide by origanum vulgare leaf plant extract: catalytic activity in the reduction of organic Dyes and Suzuki-Miyaura cross-coupling reaction. Catal Lett 148:277–288. https://doi.org/10.1007/s10562-017-2220-4
Sheny DS, Philip D, Mathew J (2012) Rapid green synthesis of palladium nanoparticles using the dried leaf of Anacardium occidentale. Spectrochim Acta A Mol Biomol Spectrosc 91:35–38. https://doi.org/10.1016/j.saa.2012.01.063
Siddiqi KS, Husen A (2016) Green synthesis, characterization and uses of palladium/platinum nanoparticles. Nanoscale Res Lett 11:482. https://doi.org/10.1186/s11671-016-1695-z
Sigma-Aldrich (2022a) Palladium powder. https://www.sigmaaldrich.com/PT/en/product/aldrich/326666. Accessed 20 Sep 2022
Sigma-Aldrich (2022b) Palladium nanopowder. https://www.sigmaaldrich.com/PT/en/product/aldrich/686468. Accessed 20 Sep 2022
Silverstein RM, Webster FX (1997) Spectrometric identification of organic compounds. Wiley, Hoboken
Singh KR, Nayak V, Sarkar T, Singh RP (2020) Cerium oxide nanoparticles: properties, biosynthesis and biomedical application. RSC Adv 10:27194–27214. https://doi.org/10.1039/D0RA04736H
Supply Chain Deep Dive Assessment (2022) Platinum group metal catalysts
Tan Q, Du C, Sun Y et al (2014) Pd-around-CeO2–x hybrid nanostructure catalyst: three-phase-transfer synthesis, electrocatalytic properties and dual promoting mechanism. J Mater Chem A 2:1429–1435. https://doi.org/10.1039/C3TA13843G
Thirumurugan A, Aswitha P, Kiruthika C et al (2016) Green synthesis of platinum nanoparticles using Azadirachta indica – An eco-friendly approach. Mater Lett 170:175–178. https://doi.org/10.1016/j.matlet.2016.02.026
Trinh HB, Lee J, Suh Y, Lee J (2020) A review on the recycling processes of spent auto-catalysts: towards the development of sustainable metallurgy. Waste Manag 114:148–165. https://doi.org/10.1016/j.wasman.2020.06.030
Tuo Y, Liu G, Dong B et al (2017) Microbial synthesis of bimetallic PdPt nanoparticles for catalytic reduction of 4-nitrophenol. Environ Sci Pollut Res 24:5249–5258. https://doi.org/10.1007/s11356-016-8276-7
Ün ŞŞ, Ünlü A, Ün İ, Ok S (2021) Green synthesis, characterization and catalytic activity evaluation of palladium nanoparticles facilitated by Punica granatum peel extract. Inorg. Nano-Met Chem 51:1232–1240. https://doi.org/10.1080/24701556.2020.1832118
Veljkovic B, Djordjevic N, Dolicanin Z et al (2018) Antioxidant and anticancer properties of leaf and fruit extracts of the wild raspberry (Rubus idaeus L.). Not Bot Horti Agrobot Cluj-Napoca 47:359–367
Wagner T, Eglinger J (2021) particlesizer: v1.0.9. ImageJ plugin to derive number based size distributions based on recorded TEM images
Wang Y, Wang S, Wang X (2009) CeO2 promoted electro-oxidation of formic acid on Pd∕C nano-electrocatalysts. Electrochem Solid-State Lett 12:B73. https://doi.org/10.1149/1.3086263
Wang Q, Cui X, Chen J et al (2012) Well-dispersed palladium nanoparticles on graphene oxide as a non-enzymatic glucose sensor. RSC Adv 2:6245. https://doi.org/10.1039/c2ra20425h
Wang L, Lin X, Zhang J et al (2019) Extraction methods for the releasing of bound phenolics from Rubus idaeus L. leaves and seeds. Ind Crops Prod 135:1–9. https://doi.org/10.1016/j.indcrop.2019.04.003
Wei X, Liu C, Cao H et al (2019) Understanding the features of PGMs in spent ternary automobile catalysts for development of cleaner recovery technology. J Clean Prod 239:118031. https://doi.org/10.1016/j.jclepro.2019.118031
Wicaksono WP, Kadja GTM, Amalia D et al (2020) A green synthesis of gold–palladium core–shell nanoparticles using orange peel extract through two-step reduction method and its formaldehyde colorimetric sensing performance. Nano-Struct Nano-Objects 24:100535. https://doi.org/10.1016/j.nanoso.2020.100535
Wiecka Z, Rzelewska-Piekut M, Regel-Rosocka M (2022) Recovery of platinum group metals from spent automotive converters by leaching with organic and inorganic acids and extraction with quaternary phosphonium salts. Sep Purif Technol 280:119933. https://doi.org/10.1016/j.seppur.2021.119933
Wongsawa T, Traiwongsa N, Pancharoen U, Nootong K (2020) A review of the recovery of precious metals using ionic liquid extractants in hydrometallurgical processes. Hydrometallurgy 198:105488. https://doi.org/10.1016/j.hydromet.2020.105488
Yakoumis I, Moschovi AM, Giannopoulou I, Panias D (2018) Real life experimental determination of platinum group metals content in automotive catalytic converters. IOP Conf Ser Mater Sci Eng 329:012009. https://doi.org/10.1088/1757-899X/329/1/012009
Yakoumis I, Panou M, Moschovi AM, Panias D (2021) Recovery of platinum group metals from spent automotive catalysts: a review. Clean Eng Technol 3:100112. https://doi.org/10.1016/j.clet.2021.100112
Zhang L, Xu Z (2018) A critical review of material flow, recycling technologies, challenges and future strategy for scattered metals from minerals to wastes. J Clean Prod 202:1001–1025. https://doi.org/10.1016/j.jclepro.2018.08.073
Zhang Y, Grass ME, Habas SE et al (2007) One-step polyol synthesis and langmuir−blodgett monolayer formation of size-tunable monodisperse rhodium nanocrystals with catalytically active (111) surface structures. J Phys Chem C 111:12243–12253. https://doi.org/10.1021/jp073350h
Zhang D, Ma X, Gu Y et al (2020) Green synthesis of metallic nanoparticles and their potential applications to treat cancer. Front Chem 8:799. https://doi.org/10.3389/fchem.2020.00799
Zheng H, Ding Y, Wen Q et al (2021) Separation and purification of platinum group metals from aqueous solution: Recent developments and industrial applications. Resour Conserv Recycl 167:105417. https://doi.org/10.1016/j.resconrec.2021.105417
Acknowledgements
This study received Portuguese national funds from FCT—Foundation for Science and Technology through projects UIDB/04326/2020, UIDP/04326/2020 and LA/P/0101/2020, and also by national funds from FCT co-financed by the Algarve´s Regional Operational Program (CRESC Algarve 2020) through Portugal 2020 and European Regional Development Fund (FEDER), under the project METALCHEMBIO (no. 29251). Moreover, this work was carried out in part using the Structural and Analytical Chemistry Platform of CCMAR (for metal analysis), for which we especially thank Dr. Vera Almeida Gomes for helping with metal analysis and Isabel Marín-Beltrán for helping with FTIR analysis. Plus, we acknowledge Prof. Ana Paula Paiva and Dr. Carlos Nogueira for providing the automobile catalytic converter leachates.
Funding
Open access funding provided by FCT|FCCN (b-on). This study received Portuguese national funds from FCT—Foundation for Science and Technology through projects UIDB/04326/2020, UIDP/04326/2020 and LA/P/0101/2020, and also by national funds from FCT co-financed by the Algarve´s Regional Operational Program (CRESC Algarve 2020) through Portugal 2020 and European Regional Development Fund (FEDER), under the project METALCHEMBIO (no. 29251).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by AN, JDC and MCC. The first draft of the manuscript was written by AN, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nobahar, A., Carlier, J.D. & Costa, M.C. Recovery of catalytic metals from leaching solutions of spent automotive catalytic converters using plant extracts. Clean Techn Environ Policy 25, 2707–2726 (2023). https://doi.org/10.1007/s10098-023-02523-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10098-023-02523-1