Abstract
S. aureus bacteremia is associated with high mortality. The aim was to identify predictors of mortality among patients with S. aureus bacteremia and evaluate the role of early source control. This retrospective study was conducted at the Lausanne University Hospital, Switzerland. All episodes of S. aureus bacteremia among adult patients from 2015 to 2021 were included. During the study period, 839 episodes of S. aureus bacteremia were included, of which 7.9% were due to methicillin-resistant isolates. Bacteremias were related to bone or joint infections (268; 31.9%), followed by bacteremia of unknown origin (158; 18.8%), proven endocarditis (118; 14.1%) and lower-respiratory tract infections (79; 9.4%). Overall 28-day mortality was 14.5%. Cox multivariate regression model showed that Charlson comorbidity index > 5 (P < 0.001), nosocomial bacteremia (P 0.019), time to blood culture positivity ≤ 13 h (P 0.004), persistent bacteremia for ≥ 48 h (P 0.004), sepsis (P < 0.001), bacteremia of unknown origin (P 0.036) and lower respiratory tract infection (P < 0.001) were associated with 28-day mortality, while infectious diseases consultation within 48 h from infection onset (P < 0.001) was associated with better survival. Source control was warranted in 575 episodes and performed in 345 episodes (60.0%) within 48 h from infection onset. Results from a second multivariate analysis confirmed that early source control (P < 0.001) was associated with better survival. Mortality among patients with S. aureus bacteremia was high and early source control was a key determinant of outcome. Infectious diseases consultation within 48 h played an important role in reducing mortality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Staphylococcus aureus is one of the most common causes of community and hospital-acquired bacteremias [1]. Due to its complexity, a holistic approach incorporating diagnostic workup (follow-up blood cultures, echocardiography, metastatic foci identification) and management (antimicrobial treatment and source control) is needed to improve outcome [2,3,4]. Despite such an approach, mortality remains high, ranging from 21 to 42% [5,6,7,8,9,10].
Several factors have been associated with worst outcome among patients with S. aureus bacteremia, such as age, comorbidities [5, 6, 9,10,11], presence of sepsis or septic shock [6, 9, 12, 13], immunosuppression [14, 15], and specific foci of infection, such as pneumonia, endocarditis or bacteremia of unknown origin [5, 8, 9, 11]. Although, aforementioned factors are unmodifiable, management of bacteremia can also impact outcome; appropriate antimicrobial treatment was repeatedly shown to improve outcome [6, 7, 16]. Source control is also a key step in early management of infected patients; however, controversy exists concerning the rapidity of source control achievement, with some studies showing an improved survival [6, 7], while in others early source control did not confer significant survival benefit [2, 16].
The aim of the present study was to identify predictors of mortality in patients with S. aureus bacteremia and evaluate the role of source control in a Swiss tertiary university hospital.
Materials and methods
We conducted a retrospective study at the Lausanne University Hospital, Switzerland during a seven-year period (2015–2021). The Lausanne University Hospital is a 1100-bed primary and tertiary care hospital with 35 intensive care units (ICU) beds. The study was approved by the ethic committee of the Canton of Vaud (CER-VD 2021–02,516) that waived the need for informed consent allowing the inclusion of all hospitalized patients except those who refused the use of their clinical and laboratory data.
Inclusion criteria were adult patients (≥ 18 years old) and presence of at least one blood culture for S. aureus (database of the microbiology laboratory). Exclusion criteria were patients’ written refusal of the use of their data and incomplete medical files (patients transferred to other hospital upon infection onset without follow-up information).
Blood cultures were incubated the BacT/ALERT System (bioMerieux, Marcy l'Etoile, France). Matrix-assisted laser desorption-ionization time of flight mass spectrometry (MALDI-TOF MS; Bruker Daltonics, Bremen, Germany) was used for the identification to the species level. Susceptibility results were collected from the microbiology laboratory database and evaluated according the EUCAST criteria [17].
Twenty-eight-day mortality was the primary outcome. Data regarding demographics (age, sex), comorbidities, Charlson Comorbidity Index [18], laboratory results (white blood cells, platelets, C-reactive protein, procalcitonin) on the day of first positive blood culture, Sequential Organ Failure Assessment (SOFA) score [19], antimicrobial treatment, source control, presence of sepsis or septic shock, infection site were retrieved from patients’ electronic health records. All data were collected, stored and managed using REDCap by an infectious diseases specialist. REDCap electronic data capture tools is hosted at Lausanne University Hospital. REDCap (Research Electronic Data Capture) is a secure, web-based software platform designed to support data capture for research studies [20, 21].
The date of collection of the first positive blood culture was defined as infection onset. A new episode was included if more than 30 days had elapsed since the first positive blood culture. Since 2007, an infectious diseases consultation was performed on a mandatory basis within the same day of S. aureus blood culture positivity [3].
Bacteremia was characterized as community if the first positive blood culture was drawn upon hospital admission or within 48 h after hospital admission and nosocomial if the first positive blood cultures were drawn after 48 h from hospital admission. Sepsis or septic shock was defined according to definition proposed by the Sepsis-3 International Consensus [22]. Complicated bacteremia was defined as presence of endocarditis, metastatic infection, implanted prostheses or persistent bacteremia for more than 48 h. Infectious endocarditis was defined according to the modified Duke criteria [23]. Cardiac predisposing factors for endocarditis were defined as cardiac conditions at high or moderate risk for infectious endocarditis [24]. Infection site was defined by the infectious diseases consultant responsible of the case on the basis of clinical, radiological, microbiological, and operative findings. Appropriate antimicrobial treatment was defined as one that included an antimicrobial agent with in vitro activity against the infecting isolate, initiated within 24 h from the infection onset, at an adequate dosage. Source control considered as warranted was (1) removal of venous catheter in patients with bacteremia of unknown origin in the presence of vascular catheter or catheter-related bacteremia; (2) surgical or imaging-guided drainage of infected collections (abscess, peritonitis, and empyema); (3) joint fluid drainage (arthrotomy or arthroscopy); (4) cardiac surgery in endocarditis patients when indicated [23]; (5) correction of urinary-tract obstruction. Early source control was defined if performed within 48 h from infection onset.
SPSS version 26.0 (SPSS, Chicago, IL, USA) and R version 4.1.3 (2022, Vienna, Austria) statistical soft wares were used for data analysis. Categorical variables were analyzed using the chi-square or Fisher exact test and continuous variables with Mann–Whitney U test. Univariate logistic regression models were assessed with 28-mortality as dependent variable. Covariates were tested for multi-collinearity through variance inflation factor assessment: those not collinear and clinically relevant were used in multivariate analysis. After checking Cox assumptions, two multivariate Cox proportional hazards regression models were performed with 28-day mortality as the time-to-event: (i) first including all patients, (ii) second assessing only patients for whom a source control was needed based on the type of the infection. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to evaluate the strength of any association. All statistic tests were 2-tailed and P < 0.05 was considered statistically significant. We finally performed Kaplan–Meier curves of the survival probability of patients with S. aureus bacteremia according to appropriate source control with 48 h from infection onset and presence of septic shock. Since it was previously suggested that source control could be influenced by care withdrawal [25], Kaplan–Meier curve was performed among patients that were alive and in maximal care for 7 days after infection onset in order to assess the role of early source control on survival.
Results
A total of 1156 episodes of S. aureus bacteremia were identified; 839 episodes in 779 patients were included (Fig. 1). Forty-seven patients had multiple episodes (41, 5, and 1 patients had 2, 3, and 4 episodes, respectively). The 60 subsequent episodes of bacteremia, occurred at a median of 8 months from the previous episode (range 1–73 months). Overall, 66 (7.9%) isolates were resistant to methicillin. Seventy-seven (9.2%) episodes were polymicrobial. Most bacteremias were related to bone and joint infections (268; 31.9%), followed by bacteremia of unknown origin (158; 18.8%), proven endocarditis (118; 14.1%), lower-respiratory tract (79; 9.4%), and central catheter (78; 9.3%). Among episodes with endocarditis, 105 had valvular infection (cardiac surgery in 36 patients among 56 with indication) and 21 lead infections of cardiovascular implantable electronic devices (CIEDs; CIED removal in 20 patients).
Overall 28-day mortality rate was 14.5% (122 episodes). Results of univariate analysis for predictors of 28-day mortality are shown in Table 1. Sepsis occurred in 352 (42.0%) episodes. Antimicrobial treatment was initiated within 24 h in 801 (95.5%) episodes and was appropriate in 761 (90.7%) episodes. Infectious diseases consultation was provided in 727 (86.7%) cases within 48 h from infection onset. Results from Cox multivariate regression model showed that Charlson comorbidity index > 5 (P < 0.001; OR 4.98, CI 2.61–9.48), nosocomial bacteremia (P 0.019; OR 1.57, CI 1.08–2.29), time to blood culture positivity ≤ 13 h (P 0.004; OR 1.85, CI 1.22–2.81), persistent bacteremia for ≥ 48 h (P 0.004; OR 1.83, CI 1.22–2.76), sepsis (P < 0.001; OR 3.39, CI 1.97–5.83), bacteremia of unknown origin (P 0.036; OR 1.64, CI 1.03–2.60) and lower respiratory tract infection (P < 0.001; OR 2.96, CI 1.77–4.95) were associated with 28-day mortality, while infectious diseases consultation within 48 h from infection onset (P < 0.001; OR 0.45, CI 0.30–0.69) was associated with better survival.
Source control was warranted in 575 (68.5%) episodes and performed in 533 (92.7%); early source control was performed in 345 (60.0%) episodes. Table 2 shows the source control procedures warranted and those performed depending on infection site. Among the 575 episodes, 28-day mortality was 12.3%. Results from a second Cox multivariate regression model (Table 3) confirmed that Charlson comorbidity index > 5 (P < 0.001; OR 5.55, CI 2.65–11.62), nosocomial bacteremia (P < 0.001; OR 3.00, CI 1.75–5.14) and sepsis (P < 0.001; OR 5.46, CI 3.06–9.71) were associated with increased 28-day mortality, while infectious diseases consultation within 48 h from infection onset (P 0.002; OR 0.39, CI 0.22–0.71) and early source control (P < 0.001; OR 0.35, CI 0.20–0.60) were associated with better survival.
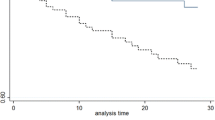
Figure 2 shows Kaplan–Meier curves of the survival probability of episodes with S. aureus according to early source control (A) in 575 episodes for which source control was warranted, (B) in episodes without septic shock, (C) with septic shock. Early source control was associated with better outcome in all episodes (P < 0.001) and in the subgroups of patients without (P 0.009) and with septic shock (P 0.035). Figure 2D shows the Kaplan–Meier curve among 555 episodes in patients that were alive and in maximal care for 7 days after infection onset; early source control was associated with better outcome (P 0.007).
Kaplan–Meier curves of the survival probability of patients with S. aureus according to early source control A in 575 episodes for which source control was warranted, B in episodes without septic shock, C with septic shock. D The Kaplan–Meier curve among 555 patients that were alive and in maximal care for 7 days after infection onset. Red line: no early source control, green line: early source control
Discussion
The present study assessing factors associated with mortality among patients with S. aureus bacteremia highlights the crucial role of early interventions, such as source control and infectious diseases consultation on the management of such bacteremic patients.
The reported 28-day mortality rate was 14.5% which is lower than that reported in the literature (21–42%) [5,6,7,8,9,10]. One hypothesis of the increased mortality among aforementioned studies could be due to the higher rate of MRSA as compared to the present study (7.9%) [6,7,8,9,10]; the rate of MRSA in the present study is comparable to that reported from S. aureus surgical site infections from a multicenter Swiss study [26].
Charlson comorbidity index, as expected, was independently associated with a worse outcome [5, 6, 9,10,11]. Both immunosuppression and septic shock are known to impact mortality of S. aureus bacteremia [9, 14, 15]. Since, the vast majority of patients received appropriate empiric antibiotic treatment (90.7%), we could not assess its impact on survival; in previous studies, a smaller percentage of patients received appropriate antimicrobial treatment during the first 24 h (52.8–74.7%), and its administration was associated with better outcome [6, 7, 16].
In accordance to the literature, persistent bacteremia was found to independently predict mortality [5, 27]. In a previous study focusing on duration of persistent bacteremia, mortality increased by 16% for every day of delay in clearance of bacteremia [27]. In that report, delay or absence of source control was associated with persistence of bacteremia [27].
As previously shown, focus of infection plays an important role on outcome, with bacteremias of unknown origin [5, 8, 9, 11] or due to pulmonary infections [8, 11,12,13] being associated with worse outcomes. In contrast to prior studies, endocarditis was not associated with increased mortality [5, 8, 9]. An explanation for the absence of such association might be the high rates of cardiac surgery (34.3%) among patients with valvular endocarditis in our setting as compared to previous studies (15–26%) [28, 29]. Both previous studies showed that absence of cardiac surgery among patients with endocarditis was associated with worse outcome [28, 29].
The role of early source control on survival among patients with S. aureus bacteremia remains debated, since some studies have shown that source control improved survival [6, 7], while in others source control did not confer significant survival benefit [2, 9, 16, 30]. Achieving adequate source control accelerates S. aureus bacteremia’s clearance and reduces associated mortality [27]. This essential element of management was not included in the analysis of mortality of many previous studies [8, 10, 11, 14, 15, 31]. There are many confounders for the association of infection source eradication and improved survival, such as decision of care limitation or withdrawal and desistance of surgeon or interventional radiologist to perform the intervention [25]. To account for such a bias, a secondary analysis was performed by including patients that were on maximal care for the first 7 days from infection onset; early source control was associated with better outcome in that subgroup, underlining the importance of prompt control of infection focus on the management of S. aureus bacteremia. Our results are in accordance to studies focusing on early source control procedures among other types of infections such us intra-abdominal infections, necrotizing fasciitis and sepsis [32,33,34]. The timing of source control was different in the present study depending on site of infection and the complexity of each intervention, with catheter removal among patients with unknown origin or catheter-related bacteremia being performed in the majority of patients within 48 h from infection onset, while drainage of abscesses or replacement of prosthetic material being less commonly performed in the same timepoint.
The management of patients with S. aureus bacteremia is complex and our results show that infectious diseases consultation within 48 h played an important role in reducing mortality. As it was shown in a previous study from our institution (2001–2010), after the implementation of mandatory infectious diseases consultation for MRSA bacteremia, rates of source control increased leading to improved survival [3]. Infectious diseases consultation was shown to increase adherence to guidelines (follow-up blood culture, echocardiography) and improve management (appropriate antimicrobial treatment, source control) and outcome [12, 31].
The present study has several limitations. First, it is a single center retrospective study, which can make the identification of the source of S. aureus bacteremia difficult for some patients. It was conducted in a setting of low MRSA prevalence meaning that results cannot be extrapolated to settings with much higher prevalence. Our cohort represents the most complex cases, since we collected data from patients requiring hospitalization in a tertiary hospital; thus, our epidemiology may not reflect non-tertiary hospitals. Second, no molecular investigation, such exotoxin genes or clonal types, was performed; this area remains scarcely investigated and more research is needed to ascertain the role of superantigens, cytotoxic exotoxins, or various clones on mortality. Additionally, since no clonal types investigation was performed, we could not ascertain if the 60 recurring episodes were from the same or a new S. aureus strain.
In conclusion, we found that in patients with S. aureus bacteremia delaying source control interventions was associated with worse outcome. Moreover, this study underscores the importance of infectious diseases consultation by guiding antimicrobial treatment, diagnostic investigations and proposing source control interventions.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, Gosbell IB (2012) Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev 25(2):362–386
Nagao M, Yamamoto M, Matsumura Y, Yokota I, Takakura S, Teramukai S, Ichiyama S (2017) Complete adherence to evidence-based quality-of-care indicators for Staphylococcus aureus bacteremia resulted in better prognosis. Infection 45(1):83–91
Tissot F, Calandra T, Prod’hom G, Taffe P, Zanetti G, Greub G, Senn L (2014) Mandatory infectious diseases consultation for MRSA bacteremia is associated with reduced mortality. J Infect 69(3):226–234
Papadimitriou-Olivgeris M, Monney P, Mueller L, Senn L, Guery B (2022) The LAUsanne STAPHylococcus aureus ENdocarditis (LAUSTAPHEN) score: A prediction score to estimate initial risk for infective endocarditis in patients with. S aureus bacteremia. Front Cardiovasc Med 9:961579
Willekens R, Puig-Asensio M, Suanzes P, Fernandez-Hidalgo N, Larrosa MN, Gonzalez-Lopez JJ, Rodriguez-Pardo D, Pigrau C, Almirante B (2021) Mortality in Staphylococcus aureus bacteraemia remains high despite adherence to quality indicators: secondary analysis of a prospective cohort study. J Infect 83(6):656–663
Marchaim D, Kaye KS, Fowler VG, Anderson DJ, Chawla V, Golan Y, Karchmer AW, Carmeli Y (2010) Case-control study to identify factors associated with mortality among patients with methicillin-resistant Staphylococcus aureus bacteraemia. Clin Microbiol Infect : Off Publ Eur Soc Clin Microbiol Infect Dis 16(6):747–752
Kim SH, Park WB, Lee KD, Kang CI, Kim HB, Oh MD, Kim EC, Choe KW (2003) Outcome of Staphylococcus aureus bacteremia in patients with eradicable foci versus noneradicable foci. Clin Infect Dis : An Off Publ Infect Dis Soc Am 37(6):794–799
Kaasch AJ, Barlow G, Edgeworth JD, Fowler VG Jr, Hellmich M, Hopkins S, Kern WV, Llewelyn MJ, Rieg S, Rodriguez-Bano J, Scarborough M, Seifert H, Soriano A, Tilley R, Torok ME, Weiss V, Wilson AP, Thwaites GE, Isac ISU, Colleagues, (2014) Staphylococcus aureus bloodstream infection: a pooled analysis of five prospective, observational studies. J Infect 68(3):242–251
Bassetti M, Peghin M, Trecarichi EM, Carnelutti A, Righi E, Del Giacomo P, Ansaldi F, Trucchi C, Alicino C, Cauda R, Sartor A, Spanu T, Scarparo C, Tumbarello M (2017) Characteristics of Staphylococcus aureus Bacteraemia and Predictors of Early and Late Mortality. PLoS One 12(2):e0170236
Austin ED, Sullivan SS, Macesic N, Mehta M, Miko BA, Nematollahi S, Shi Q, Lowy FD, Uhlemann AC (2020) Reduced Mortality of Staphylococcus aureus Bacteremia in a Retrospective Cohort Study of 2139 Patients: 2007–2015. Clin Infect Dis : An Off Publ Infect Dis Soc Am 70(8):1666–1674
Nambiar K, Seifert H, Rieg S, Kern WV, Scarborough M, Gordon NC, Kim HB, Song KH, Tilley R, Gott H, Liao CH, Edgeworth J, Nsutebu E, Lopez-Cortes LE, Morata L, Walker AS, Thwaites G, Llewelyn MJ, Kaasch AJ, International Staphylococcus aureus collaboration study g, the ESGfBI, Sepsis (2018) Survival following Staphylococcus aureus bloodstream infection: A prospective multinational cohort study assessing the impact of place of care. J Infect 77(6):516–525
Papadimitriou-Olivgeris M, Portillo V, Kampouri EE, Nusbaumer C, Monnerat LB, Duplain H (2020) Impact of universal infectious diseases consultation on the management of Staphylococcus aureus bloodstream infection in a Swiss community hospital. Diagn Microbiol Infect Dis 97(1):115001
Katsarou I, Paraskevopoulou NM, Papadimitriou-Olivgeris M, Giormezis N, Militsopoulou M, Kolonitsiou F, Marangos M, Anastassiou ED, Spiliopoulou I (2020) Fatality of Staphylococcus aureus infections in a Greek university hospital: role of inappropriate empiric treatment, methicillin resistance, and toxin genes’ presence. Eur J Clin Microbiol Infect Dis : Off Publ Eur Soc Clin Microbiol 39(3):443–450
Forsblom E, Ruotsalainen E, Molkanen T, Ollgren J, Lyytikainen O, Jarvinen A (2011) Predisposing factors, disease progression and outcome in 430 prospectively followed patients of healthcare- and community-associated Staphylococcus aureus bacteraemia. J Hosp Infect 78(2):102–107
Kaech C, Elzi L, Sendi P, Frei R, Laifer G, Bassetti S, Fluckiger U (2006) Course and outcome of Staphylococcus aureus bacteraemia: a retrospective analysis of 308 episodes in a Swiss tertiary-care centre. Clin Microbiol Infect : Off Publ Eur Soc Clin Microbiol Infect Dis 12(4):345–352
Gasch O, Camoez M, Dominguez MA, Padilla B, Pintado V, Almirante B, Molina J, Lopez-Medrano F, Ruiz E, Martinez JA, Bereciartua E, Rodriguez-Lopez F, Fernandez-Mazarrasa C, Goenaga MA, Benito N, Rodriguez-Bano J, Espejo E, Pujol M, Groups RGS (2013) Predictive factors for mortality in patients with methicillin-resistant Staphylococcus aureus bloodstream infection: impact on outcome of host, microorganism and therapy. Clin Microbiol Infect : Off Publ Eur Soc Clin Microbiol Infect Dis 19(11):1049–1057
The European Committee on Antimicrobial Susceptibility Testing (2022) Breakpoint tables for interpretation of MICs and zone diameters. Version 12.0. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_12.0_Breakpoint_Tables.pdf. Accessed 01 Dec 2022
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonca A, Bruining H, Reinhart CK, Suter PM, Thijs LG (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure On behalf of the Working. Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22(7):707–710
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG (2009) Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2):377–381
Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, Duda SN, Consortium RE (2019) The REDCap consortium: building an international community of software platform partners. J Biomed Inform 95:103208
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315(8):801–810
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL, Group ESCSD (2015) 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36(44):3075–3128
Dajani AS, Taubert KA, Wilson W, Bolger AF, Bayer A, Ferrieri P, Gewitz MH, Shulman ST, Nouri S, Newburger JW, Hutto C, Pallasch TJ, Gage TW, Levison ME, Peter G, Zuccaro G Jr (1997) Prevention of bacterial endocarditis: recommendations by the American Heart Association. Clin Infect Dis : An Off Publ Infect Dis Soc Am 25(6):1448–1458
Damonti L, Erard V, Garbino J, Schrenzel J, Zimmerli S, Muhlethaler K, Imhof A, Zbinden R, Fehr J, Boggian K, Bruderer T, Fluckiger U, Frei R, Orasch C, Conen A, Khanna N, Bregenzer T, Bille J, Lamoth F, Marchetti O, Bochud PY, Fungal Infection Network S, of Switzerland (2017) Catheter retention as a consequence rather than a cause of unfavorable outcome in candidemia. Intensive Care Med 43(6):935–939
Abbas M, Aghayev E, Troillet N, Eisenring MC, Kuster SP, Widmer AF, Harbarth S, SwissNoso, (2018) Temporal trends and epidemiology of Staphylococcus aureus surgical site infection in the Swiss surveillance network: a cohort study. J Hosp Infect 98(2):118–126
Minejima E, Mai N, Bui N, Mert M, Mack WJ, She RC, Nieberg P, Spellberg B, Wong-Beringer A (2020) Defining the breakpoint duration of Staphylococcus aureus bacteremia predictive of poor outcomes. Clin Infect Dis : An Off Publ Infect Dis Soc Am 70(4):566–573
Asgeirsson H, Thalme A, Kristjansson M, Weiland O (2015) Incidence and outcome of Staphylococcus aureus endocarditis–a 10-year single-centre northern European experience. Clin Microbiol Infect : Off Publ Eur Soc Clin Microbiol Infect Dis 21(8):772–778
Miro JM, Anguera I, Cabell CH, Chen AY, Stafford JA, Corey GR, Olaison L, Eykyn S, Hoen B, Abrutyn E, Raoult D, Bayer A, Fowler VG, Jr., International Collaboration on Endocarditis Merged Database Study G (2005) Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clinical Infect Dis : An Off Publ Infect Dis Soc Am 41(4):507–514
Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ (2002) Health care–associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137(10):791–797
Rieg S, Peyerl-Hoffmann G, de With K, Theilacker C, Wagner D, Hubner J, Dettenkofer M, Kaasch A, Seifert H, Schneider C, Kern WV (2009) Mortality of S. aureus bacteremia and infectious diseases specialist consultation-a study of 521 patients in Germany. J Infect 59(4):232–239
Reitz KM, Kennedy J, Li SR, Handzel R, Tonetti DA, Neal MD, Zuckerbraun BS, Hall DE, Sperry JL, Angus DC, Tzeng E, Seymour CW (2022) Association between time to source control in sepsis and 90-day mortality. JAMA Surg 157(9):817–826
Boyer A, Vargas F, Coste F, Saubusse E, Castaing Y, Gbikpi-Benissan G, Hilbert G, Gruson D (2009) Influence of surgical treatment timing on mortality from necrotizing soft tissue infections requiring intensive care management. Intensive Care Med 35(5):847–853
Azuhata T, Kinoshita K, Kawano D, Komatsu T, Sakurai A, Chiba Y, Tanjho K (2014) Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Crit Care 18(3):R87
Funding
Open access funding provided by University of Lausanne The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
BG and LS conceived the idea. MPO and GC collected the patients’ data. BG supervised the project. MPO and GC performed the analysis and interpreted the results. MPO wrote the manuscript. All authors contributed to manuscript revision and read and approved the submitted version.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the ethics committee of the Canton of Vaud (CER-VD 2021–02516).
Consent to participate
Due to the retrospective nature of the study, the ethics committee waived the need of informed consent to participate.
Consent for publication
Due to the retrospective nature of the study, the ethics committee waived the need of informed consent to publish.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Papadimitriou-Olivgeris, M., Caruana, G., Senn, L. et al. Predictors of mortality of Staphylococcus aureus bacteremia among patients hospitalized in a Swiss University Hospital and the role of early source control; a retrospective cohort study. Eur J Clin Microbiol Infect Dis 42, 347–357 (2023). https://doi.org/10.1007/s10096-023-04557-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-023-04557-1