Abstract
The objective was to describe the epidemiology, bacteriology, clinical presentation, risk factors for endocarditis (IE), diagnostic workup, and outcome of patients with bacteremia caused by the non-influenzae Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, and Kingella genera (HACEK). A retrospective population-based cohort of patients with bacteremia collected from 2012 to 2017 was identified. Clinical data from identified patients were collected from medical records to classify patients, calculate incidences, analyze risk factors of IE, and describe the management and outcome of the cohort. A total of 118 episodes of HACEK bacteremia were identified, of which 27 were definite IE. The incidence of HACEK bacteremia was 5.2 and of HACEK IE 1.2 episodes per 1,000,000 inhabitants per year. Other focal infections were identified in 55 of 118 of the episodes, most commonly within the abdomen (26 episodes). The propensity to cause IE ranged from 62 in Aggregatibacter actinomycetemcomitans to 6% in Eikenella. Risk factors for IE were cardiac implantable electronical device, predisposing cardiac conditions, community acquisition, long duration of symptoms, multiple positive blood cultures, fever, heart murmur, embolization, and unknown origin of infection. The scoring system DENOVA developed to predict IE in bacteremia with Enterococcus faecalis also had a high sensitivity and specificity for predicting IE in HACEK bacteremia. The 30-day mortality was 4% in IE and 15% in non-IE bacteremia, and only one case of relapse was found. IE is common in bacteremia with Aggregatibacter, Cardiobacterium, and Kingella but relatively rare in Haemophilus and Eikenella. Treatment failures are very rare, and DENOVA can be used to evaluate the need for transesophageal echocardiography.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteremia constitutes a clinical situation with a risk for different complications, of which infective endocarditis (IE) is of high relevance [1]. Faced with a positive blood culture (BC) result, the clinician has to evaluate the risk of IE and decide on the diagnostic workup and treatment. For bacteria that are common causes of IE, risk stratification systems to determine the need for transesophageal echocardiography (TEE) in bacteremia have been developed [2,3,4,5,6].
The genera of Haemophilus (except H. influenzae), Aggregatibacter, Cardiobacterium, Eikenella, and Kingella (HACEK) make up a group of gram-negative bacteria which are commensals of the oral cavity and gastrointestinal tract and are known to cause IE [7,8,9]. In a large international study, HACEK bacteria caused 1.3% of IE cases [8]. Other clinical manifestations of the HACEK are species and genus specific but they also have some general features, resulting in dental infections and bacteremia with unknown origin [7]. The risk for IE in cases of HACEK bacteremia has been shown to vary for the different HACEK genera [10]. In a previous study, all cases (18/18) of Aggregatibacter actinomycetemcomitans bacteremia represented IE, whereas no case of IE (0/11) was observed in bacteremia with Eikenella corrodens. Furthermore, bacteremia with Haemophilus parainfluenzae (10/18), Cardiobacterium (7/8), Kingella (8/19), and other species of the Aggregatibacter genus (9/13) had a very high propensity to cause IE [10]. The annual incidence of bacteremia in that study was 2.2 per million and for IE 1.4, including both definite and possible IE in the cohort (Dr Murdoch, personal communication).
A population-based study from the Danish national microbiology database focused on the epidemiology of HACEK bacteremia [11], not specifying the clinical conditions. The annual incidence of bacteremia with HACEK was found to be of 4.4 per million population. Of 147 episodes detected, 55 Haemophilus species, 37 Aggregatibacter, 9 Cardiobacterium, 21 Eikenella, and 27 cases of Kingella were found [11].
Cephalosporins are the primary treatment options in international guidelines [1, 12], but due to lower levels of resistance, the Swedish recommendations advocate ampicillin as the first choice and cefotaxime when resistance is demonstrated.
In this study, we assembled a large population-based cohort of patients with HACEK bacteremia and describe the epidemiology, clinical presentation, diagnostic workup, risk factors for IE, and outcome. We also investigate whether scoring systems to determine the need for echocardiography in patients with bacteremia caused by streptococci and enterococci were applicable also on patients with HACEK bacteremia.
Material and methods
All consecutive BCs positive for the HACEK genera from January 2012 to December 2017 were obtained from the databases of the Clinical Microbiology Laboratory in Skåne County, (the only laboratory in the region with a catchment area of 1.3 million inhabitants and nine hospitals) and from Karolinska University Laboratory, Karolinska University Hospital, Stockholm, Sweden (analyzing BC from a population of 1.9 million inhabitants in the Stockholm County).
All medical records of patients with HACEK bacteremia were studied retrospectively. Ethical approval was obtained from the Ethics Committee of Lund University (2017/1002) and from the Ethics Committee review board in Stockholm (recordal 2015/1184-31).
Microbiology
Blood culture system and incubation
The BacT/Alert culture media (aerobic, anaerobic, and pediatric) and BacT/Alert 3D incubator (bioMérieux, Durham, NC, USA) according to the manufacturer’s instructions were used during the whole study period for BC in Stockholm County. In the Skåne County, the same BacT/Alert blood culture system (bioMérieux, Durham, NC, USA) was used from 2012 to late 2014, and it was replaced by the BACTEC FX blood culture system (Becton Dickinson, Franklin Lakes, NJ, USA) using the BD BACTEC culture media (Plus Aerobic/F, Lytic/10 Anaerobic and Peds Plus/F) in December 2014, and it was used for the remaining period of the study.
Species identification
Determination of genera and species was performed with Microflex matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS) at both centers, using the direct transfer method as described previously [13]. The generated mass spectra of the bacterial isolates were analyzed with the MALDI Biotyper 4.1 software and the MALDI Biotyper Library DB-7854 (Bruker, Bremen, Germany). If a MALDI Biotyper score value of ≥ 2.0 was obtained in the routine analysis, this was considered to be reliable to the species level. In cases where a score of < 2.0 and ≥ 1.8 was found and the second-best species had a score difference greater than 0.2, the identification of the species was considered reliable as previously described [14]. In other cases, a new MALDI-TOF MS analysis was made on available stored isolates using the standard ethanol–formic acid extraction method described by the instrument manufacturer (Bruker, Bremen, Germany). The remaining isolates were analyzed by sequencing the 16S rRNA gene and assigned a species at the two centers as described [15, 16] when retrievable. In two isolates (one isolate of Haemophilus and one of Eikenella), the species determination was not successful by neither MALDI-TOF nor 16S, and the isolates were assigned only to the genus level.
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed at the clinical microbiology laboratories according to the EUCAST methodology for MIC determination using gradient tests (Etest, Oxoid) [17]. Müller–Hinton fastidious broth agar plates produced by the Substrate department at Karolinska University Hospital and by Clinical Microbiology, Labmedicin, Lund, were used. Bacterial inoculum of 0.5 McFarland and gradient test were applied on agar plates, and inhibition of growth was judged with naked eye after 18 h (± 2 h) of incubation in 35 °C (± 1 °C) in 4–6% CO2 environment.
Definitions
An episode of HACEK bacteremia was defined as a clinical situation in a patient resulting in BC taken by the decision of the treating clinician, showing growth of a HACEK isolate. All BCs with growth of HACEK bacteria were defining an episode. No episodes were excluded, assuming the finding to be a contamination. Multiple positive BCs taken on different days were included in the same episode if they were taken during the same clinical situation. To be able to discriminate BCs taken within an episode from a new episode of bacteremia, an episode was delimited by at least 7 days of effective treatment or by 30 days.
IE was defined using the modified Duke criteria [18]. With regards to the Duke criteria, the HACEK bacteria were considered “a typical microorganism consistent with IE,” so growth in two separate blood cultures was sufficient to constitute a major criterion. In cases where the major Duke criterion for echocardiography was not met, the ESC criteria were used to further confirm the diagnosis of IE using ECG-triggered cardiac CT or 18FDG-PET-CT [1]. Other focal infections were diagnosed as described [2]. If two out of three of the following findings were found, a diagnosis was considered established: (1) typical signs or symptoms of the infection; (2) imaging results indicating the diagnosis; and (3) microbiological result, other than BC, confirming the diagnosis [2].
To evaluate the scoring systems NOVA, DENOVA, and HANDOC [2, 3, 5], scoring of patients was performed using the information available to the clinician at the time when receiving the positive BC result, analyzed to the genus or species. NOVA score parameters were defined as described by Bouza et al. [3] with modifications of number of cultures as described by Dahl et al. [19], and DENOVA and HANDOC according to the publications [2, 5]. An “etiology” of Aggregatibacter, Cardiobacterium, or Kingella was given one point for the A in the calculation of the HANDOC score [5]. A relapse was defined as a BC with growth of the same genus or species within the 365 days of follow-up after an episode. Comorbidities were classified according to the Charlson index [20].
Data collection
Clinical data from each episode were collected from 365 days before its start until 365 days after the first positive BC. Thus, age, gender, comorbidities, previous bacteremia, symptoms, signs, performed radiology and its results, culture results other than blood cultures, hematuria, duration of symptoms, death within 365 days of positive culture, days hospitalized, and cultures or clinical conditions indicating therapeutic failure during follow-up were registered. Furthermore, data on intravenous drug use, other predisposing heart conditions, fever, vascular or immunological phenomena, microbiological data fulfilling or not fulfilling the prerequisites for Duke minor or major criteria, and whether transthoracic echocardiography (TTE) or TEE was performed and if diagnostic criteria for IE were met [1, 18] were collected. Missing data were registered as lack of result in that variable. No imputations were made.
Statistics
The analysis of the collected data was calculated in the statistical program Stata (StataCorp, College Station, TX, USA). The odds ratios and their confidence intervals were calculated when applicable. The χ2 test was used when applicable, and otherwise, the p value of Fisher’s exact test was used. Differences between continuous variables were analyzed with Wilcoxon’s rank-sum test. Values are presented as proportions or medians with interquartile ranges (IQR).
Results
HACEK bacteremia
The cohort constituted 3.2 million inhabitants who were studied for 7 years, and 118 episodes of HACEK bacteremia were found, resulting in an incidence of 5.3 cases per 1,000,000 and year (Table 1). Most episodes were caused by Haemophilus, Aggregatibacter, and Eikenella, while fewer episodes were caused by Cardiobacterium and Kingella (Table 1). The occurrence of IE and other focal infections for the different species and genera is shown (Table 2). Haemophilus and Eikenella episodes often had abdominal origin of infection, and mainly Haemophilus were causing urinary tract infections. Infection foci other than IE, due to hematogenous seeding of bacteria, were mainly caused by the Aggregatibacter genus (3/4). All genera had 25% or more episodes with an unknown focus (Table 2).
IE caused by HACEK
In all, 27 cases of definite IE were identified, resulting in an incidence of 1.2/106/year.
The IE episodes were 5, 14, 3, 2, and 3 in the five genera in HACEK, giving an incidence of 0.62 of Aggregatibacter IE, the genus most often causing IE (Table 1). All Haemophilus IE episodes were caused by H. parainfluenzae, and no cases of IE caused by Haemophilus haemolyticus or Haemophilus parahaemolyticus were found. The propensity to cause IE was very diverse among the genera and species (Table 1). The most prone genus to cause IE was the Aggregatibacter, Cardiobacterium, and Kingella genera (33-50% IE), while Haemophilus and Eikenella were less prone (14 and 6%). Among the species, A. actinomycetemcomitans was the most common cause of IE (8 episodes) and most prone to cause IE (62% of episodes) (Table 1). However, A. actinomycetemcomitans was the only species significantly more prone to cause IE compared with the other species in the cohort (Table 3).
Comparison of episodes of HACEK bacteremia with and without IE
A comparison of clinical characteristics of episodes with HACEK IE and non-IE is shown in Table 4. Patients with HACEK IE were slightly older and had lower Charlson comorbidity score than patients without IE, but the differences were not significant. Furthermore, a long duration of symptoms, the presence of a cardiac implantable electronical device (CIED), prosthetic heart valve or native heart valve disease, heart murmur, fever, embolization, growth in all or the majority of BC, only one species in the BC, and unknown origin of infection were all significantly more common in patients with IE (Table 4).
Possible IE
In addition to the 27 episodes of definite IE, 43 episodes fulfilled the clinical criteria for possible IE [18]. About half (21/43) of these were diagnosed with a known origin of infection, most commonly in the abdomen (Fig. 1). Eleven of these 21 were subjected to TTE or TEE (all negative) and had a median treatment time of 11 days (IQR 7–14). However, 22 had unknown origin of infection. Twelve of these 22 were treated as IE and 10 were not, with a median treatment time of 42 (IQR 31–44) and 13 (IQR 4–40), respectively. In six episodes treated as IE, a prosthetic valve was present, and in 4 episodes in the group not treated as IE (Fig. 1).
Description of episodes with possible IE based on clinical Duke criteria. aOne patient was diagnosed with two possible origins of infection during one episode. bIncludes two patients with spondylodiscitis, one with aortic graft infection, and one with septic arthritis. BC, blood culture. Major, major criterion in the Duke criteria. Minor, minor criterion in the Duke criteria. Prosthesis, valvular prosthesis
Susceptibility testing results
Of 118 isolates tested for three antibiotics (ampicillin, cefotaxime, and ciprofloxacin), two had MIC > 2 mg/L for ampicillin, one had MIC > 1 mg/L for cefotaxime, and no isolate had MIC > 0.25 mg/L for ciprofloxacin (non-susceptible according to pharmacokinetic–pharmacodynamic breakpoints for these antibiotics [17]) (Fig. 2). However, according to the EUCAST epidemiological cutoff, two of the Kingella isolates were resistant to ampicillin using the cutoff at 0.06 mg/L [22] (Fig. 2).
Distribution of MIC values for ampicillin (a), cefotaxime (b), and ciprofloxacin (c) for the isolates shown for each HACEK genus. The dashed lines represent the suggested cutoff, above which the isolate is regarded as resistant, according to EUCAST. The upper dashed line represents the EUCAST PK/PD breakpoints [21]. a Ampicillin 2 mg/L. b Cefotaxime 1 mg/L. c Ciprofloxacin 0.25 mg/L. The lower dashed line in column K represents the susceptibility break points for Kingella [22]. a Ampicillin 0.06 mg/L. b Cefotaxime 0.125 mg/L. c Ciprofloxacin 0.06 mg/L
Performance of scoring systems
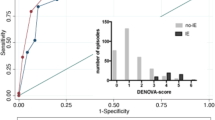
The sensitivity and specificity to separate cases of HACEK IE from non-IE of the scoring systems, NOVA, DENOVA, and HANDOC, were tested. The data are shown in Supplementary table 1, and three ROC curves were constructed (Fig. 3). The area under the curve (AUC) for the three scoring systems was 0.84, 0.92, and 0.89, respectively. The DENOVA score had a significantly larger AUC than the NOVA score, and the AUC for the HANDOC score was intermediate (Supplementary table 1). At the predefined cutoffs, all scoring systems had a high sensitivity, ≥ 0.92 (Table 5), but the specificities were diverse, with 0.23, 0.79, and 0.49, respectively. That makes high negative predictive values for all scores but positive predictive value of 0.28, 0.57, and 0.36, respectively (Table 5).
Treatment and investigations
The median treatment duration was 35 days in IE episodes and 12 days in non-IE (Table 6). In most episodes, beta-lactam antibiotics were chosen for the entire treatment time, but in 39 episodes (five with IE), the treatment was changed to ciprofloxacin. Ciprofloxacin was given for a median time of 10 days (IQR 5–17) in the whole cohort and for 38 days (IQR 28–60) in the IE episodes.
TTE or TEE was performed in all episodes of IE and in 53% of the non-IE episodes. TEE was done in 93% of IE episodes and 36% of non-IE episodes (Table 5). One patient was investigated using FDG-PET-CT, whereas no cardiac CT investigations were performed.
Outcome
Only one relapse was identified during the follow-up time, and this was in a patient with growth of Cardiobacterium in one out of four BC bottles, prosthetic valve, fever, and a cerebral stroke that was suspected to be IE. The patient was subjected to transthoracic echocardiography, without IE findings, but not to TEE, and was given 4 days of ampicillin. After 180 days, the patient had a new stroke and was found to have IE.
The mortality, within 30 and 365 days, was lower in the patients with episodes of IE than in non-IE (4% and 4% compared with 15% and 20%, respectively), but the difference did not reach significance (Table 6).
Patients that died were significantly older, median 75 years (IQR 57–84), than the rest of the cohort, 60 years (IQR 39–73) (p value: 0.004), and had a higher Charlson score (median 4 (IQR 2–8) and 1 (IQR 0–2), respectively, p = value: < 0.001) (data not shown). The one diseased IE patient died of a massive intracranial hemorrhage. Thirteen patients had metastasized cancer or hematological cancer. Of the remaining five diseased patients, all died within 30 days. Three had polymicrobial infections, one was a 96-year-old woman diagnosed with pneumonia, one had an abdominal infection, and the third had a wound infection in connection with severe vascular insufficiency. Two patients had monomicrobial infections, one was a 91-year-old woman diagnosed with pneumonia after operation of a broken femur and one was admitted with massive intracranial hemorrhage and died within 2 days.
Discussion
In this study, we describe the epidemiology, bacteriology, clinical presentation, and diagnostic workup of episodes of bacteremia of HACEK in a population-based cohort from two regions in Sweden. One of our main findings was that all the genera and species in the group had a high propensity to cause IE but that the inter-genus differences were large. This is in line with previous findings [10]. The genus of Aggregatibacter, especially the species A. actinomycetemcomitans, and Cardiobacterium hominis were most prone to cause IE, 62% and 50%, respectively (Table 1). These propensities were much higher than those of other species and genera also known to cause IE, for example 11% for Staphylococcus aureus [6], 12–13% for Enterococcus faecalis [19, 23], and 7.7% for Viridans streptococci [5].
Our study found a higher incidence of HACEK bacteremia, 5.3 episodes/106/year, compared with the previous population-based studies [10, 11]. The incidences for the individual genera and species were similar, except for A. actinomycetemcomitans and Eikenella which were higher in our study compared with the Danish one [11]. We found an annual incidence of definite IE of 1.2 episodes/106/year, which is similar to that described by Yew et al. [10]. However, in that study, both possible and definite IE were summed up to an incidence of 1.4. If the possible IE episodes in our study were added, the incidence of IE would rise to 2.2. Taken together, the three studies indicate a similar epidemiology with some local variations. Improved bacteriological diagnostics methods and an increased indication for performing blood cultures could contribute to the higher incidence in later studies.
There are no EUCAST species-specific breakpoints for HACEK bacteria except for Kingella kingae [22]. However, the interpretation of MIC values can instead be performed using the pharmacokinetic–pharmacodynamic breakpoints, or for H. parainfluenzae, H. influenzae breakpoints can be used [21]. Very few isolates in our study showed resistance to the antibiotics mainly used to treat HACEK infections. Analyzing our isolates, aware of the privileged Swedish context, there was no obvious ground to change the Swedish recommendation to use ampicillin as the empirical treatment after species determination. However, we found two isolates of Kingella with ampicillin MIC values higher than the EUCAST description of the wild type population, but lower than the pharmacokinetic–pharmacodynamic breakpoint of ampicillin [17, 21]. The lack of species-specific breakpoints makes it difficult to interpret resistance data reported for HACEK bacteria, and it would be valuable if such were developed for all HACEK species.
As expected, patients with IE had significant differences in underlying conditions and clinical presentation compared with the non-IE episodes. HACEK IE had similar risk factors as IE caused by streptococci, enterococci, and related genera [2, 5, 24]. Due to the limited number of IE cases and the high number of features associated with IE in univariable comparisons, we did not precede to make multivariable analyses. Thus, we cannot conclude which variables are independently associated with IE in HACEK bacteremia. Of interest, the presence of a CIED was correlated with IE, in contrast to multiple studies of bacterial species also known to cause IE [2, 3, 5, 24]. In fact, six out of 9 episodes of bacteremia in patients with CIED were diagnosed with IE. Aggregatibacter caused five of the six episodes of CIED-related IE, making a patient with Aggregatibacter bacteremia and a CIED highly likely to have IE.
When testing the scoring systems developed to estimate the risk of IE in bacteremia with other pathogens, DENOVA (developed for bacteremia with E. faecalis) performs best and could be used to guide the use of TEE in HACEK bacteremia. Interestingly, the two patients with IE not predicted by DENOVA to be at risk for IE had a CIED. Thus, we suggest that DENOVA could be used to guide the use of TEE in HACEK bacteremia but that TEE should always be performed in episodes of HACEK bacteremia in patients with CIED.
In our study, only one patient was subjected to FDG-PET-CT, a patient with Aggregatibacter segnis in blood cultures and an aortic graft infection. Thus, the ESC recommendations to proceed with PET-CT or cardiac CT after a negative TEE and continuous suspicion of IE [1] have not been used in our cohort. The only case reported as a relapse was likely an unfortunate missed case of IE, in whom TEE was not performed. We find it likely that the entire period of illness of this patient represents one infection episode with Cardiobacterium.
The international guidelines [1, 12] recommend antibiotic treatment for 4 weeks in native valve and 6 weeks in prosthetic valve HACEK IE. Many episodes with possible IE were treated with a shorter course than recommended for IE, with only one case of relapse. We therefore believe that cases of IE were not missed to any large extent. However, we cannot rule out that some case of missed IE was cured by a shorter course of antibiotics.
The retrospective design of our study limits the amount of information available for each patient. Only in 75 of 118 episodes a TTE or TEE was performed, and therefore, there is a risk that some episodes of IE might not have been detected. However, we consider that the number of missed IE episodes was limited because of the long follow-up period without relapse of bacteremia. Eighteen non-IE patients died during the follow-up, fourteen during the first 30 days from the positive blood culture, but only one arose suspicion of an undiagnosed IE. Only four patients died later than 30 days but within the year of observation. All these had malignancies, and all died without an apparent HACEK infection. It cannot be excluded that some of these had a relapse, though none of the deaths arose suspicion of undiagnosed infection according to our review of the medical records.
We believe that the present study contributes to the understanding of HACEK bacteremia and IE and how it should be managed. The clinician must have a high level of suspicion of IE in HACEK bacteremia, especially if caused by the genera Aggregatibacter, Cardiobacterium, and Kingella. The algorithm of DENOVA can provide additional aid in the decision in whether echocardiography should be performed.
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
References
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska-Gosciniak E, Price S, Roos-Hesselink J, Snygg-Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL, Group ESCSD (2015) 2015 ESC guidelines for the management of infective endocarditis: the task force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J 36(44):3075–3128
Berge A, Krantz A, Östlund H, Naucler P, Rasmussen M (2019) The DENOVA score efficiently identifies patients with monomicrobial Enterococcus faecalis bacteremia where echocardiography is not necessary. Infection 47(1):45–50
Bouza E, Kestler M, Beca T, Mariscal G, Rodriguez-Creixems M, Bermejo J, Fernandez-Cruz A, Fernandez-Aviles F, Munoz P (2015) The NOVA score: a proposal to reduce the need for transesophageal echocardiography in patients with enterococcal bacteremia. Clin Infect Dis 60(4):528–535
Palraj BR, Baddour LM, Hess EP, Steckelberg JM, Wilson WR, Lahr BD, Sohail MR (2015) Predicting risk of endocarditis using a clinical tool (PREDICT): scoring system to guide use of echocardiography in the management of Staphylococcus aureus bacteremia. Clin Infect Dis 61(1):18–28
Sunnerhagen T, Törnell A, Vikbrant M, Nilson B, Rasmussen M (2018) HANDOC: a handy score to determine the need for echocardiography in non-beta-hemolytic Streptococcal Bacteremia. Clin Infect Dis 66(5):693–698
Tubiana S, Duval X, Alla F, Selton-Suty C, Tattevin P, Delahaye F, Piroth L, Chirouze C, Lavigne JP, Erpelding ML, Hoen B, Vandenesch F, Iung B, Le Moing V, Group VAS (2016) The VIRSTA score, a prediction score to estimate risk of infective endocarditis and determine priority for echocardiography in patients with Staphylococcus aureus bacteremia. J Infect 72(5):544–553
Revest M, Egmann G, Cattoir V, Tattevin P (2016) HACEK endocarditis: state-of-the-art. Expert Rev Anti-Infect Ther 14(5):523–530
Chambers ST, Murdoch D, Morris A, Holland D, Pappas P, Almela M, Fernandez-Hidalgo N, Almirante B, Bouza E, Forno D, del Rio A, Hannan MM, Harkness J, Kanafani ZA, Lalani T, Lang S, Raymond N, Read K, Vinogradova T, Woods CW, Wray D, Corey GR, Chu VH, International Collaboration on Endocarditis Prospective Cohort Study I (2013) HACEK infective endocarditis: characteristics and outcomes from a large, multi-national cohort. PLoS One 8(5):e63181
Das M, Badley AD, Cockerill FR, Steckelberg JM, Wilson WR (1997) Infective endocarditis caused by HACEK microorganisms. Annu Rev Med 48:25–33
Yew HS, Chambers ST, Roberts SA, Holland DJ, Julian KA, Raymond NJ, Beardsley J, Read KM, Murdoch DR (2014) Association between HACEK bacteraemia and endocarditis. J Med Microbiol 63(Pt 6):892–895
Lutzen L, Olesen B, Voldstedlund M, Christensen JJ, Moser C, Knudsen JD, Fuursted K, Hartmeyer GN, Chen M, Sondergaard TS, Rosenvinge FS, Dzajic E, Schonheyder HC, Norskov-Lauritsen N (2018) Incidence of HACEK bacteraemia in Denmark: a 6-year population-based study. Int J Infect Dis 68:83–87
Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levison ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA (2015) Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation 132(15):1435–1486
Bizzini A, Durussel C, Bille J, Greub G, Prod'hom G (2010) Performance of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of bacterial strains routinely isolated in a clinical microbiology laboratory. J Clin Microbiol 48(5):1549–1554
Couturier MR, Mehinovic E, Croft AC, Fisher MA (2011) Identification of HACEK clinical isolates by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 49(3):1104–1106
Johansson N, Vondracek M, Backman-Johansson C, Skold MC, Andersson-Ydsten K, Hedlund J (2019) The bacteriology in adult patients with pneumonia and parapneumonic effusions: increased yield with DNA sequencing method. Eur J Clin Microbiol Infect Dis 38(2):297–304
Sonesson A, Oqvist B, Hagstam P, Bjorkman-Burtscher IM, Miorner H, Petersson AC (2004) An immunosuppressed patient with systemic vasculitis suffering from cerebral abscesses due to Nocardia farcinica identified by 16S rRNA gene universal PCR. Nephrol Dial Transplant 19(11):2896–2900
EUCAST (2020) Antimicrobial susceptibility testing. http://www.eucast.org/ast_of_bacteria/. Cited 1 Jan, 2020
Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR (2000) Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30(4):633–638
Dahl A, Lauridsen TK, Arpi M, Sorensen LL, Ostergaard C, Sogaard P, Bruun NE (2016) Risk factors of endocarditis in patients with Enterococcus faecalis bacteremia: external validation of the NOVA score. Clin Infect Dis 63(6):771–775
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
EUCAST (2020) Clinical breakpoints - bacteria (v 10.0), http://www.eucast.org/clinical_breakpoints/. Cited Accessed 1 Jan 2020
Matuschek E, Ahman J, Kahlmeter G, Yagupsky P (2018) Antimicrobial susceptibility testing of Kingella kingae with broth microdilution and disk diffusion using EUCAST recommended media. Clin Microbiol Infect 24(4):396–401
Pinholt M, Ostergaard C, Arpi M, Bruun NE, Schonheyder HC, Gradel KO, Sogaard M, Knudsen JD, Danish Collaborative Bacteraemia N (2014) Incidence, clinical characteristics and 30-day mortality of enterococcal bacteraemia in Denmark 2006-2009: a population-based cohort study. Clin Microbiol Infect 20(2):145–151
Berge A, Kronberg K, Sunnerhagen T, Nilson BHK, Giske CG, Rasmussen M (2019) Risk for endocarditis in bacteremia with Streptococcus-like bacteria: a retrospective population-based cohort study. Open Forum Infect Dis 6(10):ofz437
Acknowledgments
We acknowledge the help with laboratory database searches by Mrs. Lena Hyllebusk, with patient record administration by Mrs. Emma Söderdahl in Lund, and Dr. Aina Iversen for the help to retrieve and revise the laboratory data from Stockholm.
Funding
Open access funding provided by Karolinska Institute. This work was supported by the Swedish Government Fund for Clinical Research (ALF), the foundations of Österlund, the Skåne University Hospital, and the Royal Physiographic Society in Lund to MR. The work was also supported by the Region Stockholm (ALF grant) for AB.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design of the study. Data collection and analysis were performed by all authors. The first draft of the manuscript was written by Andreas Berge, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Ethical approval was obtained from the Ethics Committee of Lund University (2017/1002) and from the Ethics Committee review board in Stockholm (recordal 2015/1184-31).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 89 kb).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Berge, A., Morenius, C., Petropoulos, A. et al. Epidemiology, bacteriology, and clinical characteristics of HACEK bacteremia and endocarditis: a population-based retrospective study. Eur J Clin Microbiol Infect Dis 40, 525–534 (2021). https://doi.org/10.1007/s10096-020-04035-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-020-04035-y