Abstract
Chronic obstructive pulmonary disease (COPD) affects approximately 65 million people from which > 25% will require intensive care unit (ICU) admission. Ventilator-associated pneumonia (VAP) is the commonest ICU infection and results in increased morbidity/mortality and costs. The literature on the interaction between COPD and VAP is scarce and controversial. The project aimed to search the literature in order to address the following: (i) Is COPD a risk factor for VAP development? (ii) Does COPD impact the outcome of patients with VAP? (iii) Does VAP development impact the outcome of COPD patients? (iv) Does COPD impact the aetiology of VAP? Current evidence on the topic is controversial. Regarding the impact of VAP on COPD patients, the majority of the existing limited number of studies suggests that VAP development results in higher mortality and longer duration of mechanical ventilation and ICU stay. Also, the majority of the studies exploring the impact of COPD on VAP outcomes suggest that COPD is independently associated with a decrease in survival, although the number of such studies is limited. Regarding the aetiology, Pseudomonas aeruginosa is the most frequent pathogen in VAP patients with COPD. Noteworthy, one study suggests that P. aeruginosa is higher in COPD patients even in the early-onset VAP subgroup. This manuscript provides a comprehensive overview of the available literature on the interaction between COPD and VAP, highlighting the differences and limitations that may have led to controversial results, and it may act as a platform for further research with important clinical implications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ventilator-associated pneumonia (VAP) is a common complication of endotracheal intubation; it is defined as pneumonia occurring at least 48 h after initiating mechanical ventilation (MV) [1, 2]. VAP is the second most common nosocomial infection and the leading cause of death from nosocomial infections in the intensive care unit (ICU) [1]. The prevalence of VAP ranges broadly from 9 to 27%; this variability might be attributed, at least partially, to the lack of a “gold standard” for diagnosis, differences in infection control practices, different case-mix and variable underlying diseases [1, 3, 4]. Overall, VAP is associated with increased mortality and, in survivors, VAP is associated with an increase in MV duration, ICU and hospital stay [3, 5,6,7]. VAP is also one of the causes of the increase in the cost of healthcare, with estimated mean attributable costs ranging from around $11,000 [7] to 40,000 USD [8]. Reported all-cause mortality ranges widely from 20 to 50% [2]. Regarding VAP attributable mortality a meta-analysis has reported it as 13% [9]; however, it remains a controversial issue with a significant number of studies tending to ignore confounding factors, such as severity-of-illness measures over time, underlying diseases and comorbidities [10].
Chronic obstructive pulmonary disease (COPD) is characterised by an airflow limitation associated with an abnormal inflammatory response of the lungs to noxious gas particles, such as cigarette smoke or pollutants [11, 12]. According to the World Health Organization (WHO) estimates, 65 million people have moderate to severe COPD [13]. More than 3 million people died of COPD in 2005, which corresponds to 5% of all deaths globally [13]. COPD causes lung tissue destruction, and it impairs the normal repair and defence mechanism of the lungs [11, 14, 15]. COPD leads to a chronic structural damage to the lungs, which along with decreased mucosal clearance and microbiome imbalances, makes them more vulnerable to invading pathogens and to developing lower respiratory tract infections [11, 16]. During the course of COPD, it is estimated that > 25% of the patients will require ICU admission for acute exacerbations and 26–74% of these patients will receive mechanical ventilator support, resulting in increased average hospital stays and healthcare costs [17, 18]. Moreover, COPD is a common comorbidity of ICU patients admitted for other medical reasons [19].
COPD is a well-recognised risk factor for community-acquired pneumonia (CAP) development [12, 16, 20,21,22,23]; however, the relationship between COPD and VAP remains controversial and underexplored. In this manuscript, the recent findings in the literature will be analysed, while also highlighting the differences and limitations which may have influenced the results. The relationship between COPD and VAP will be discussed by following the order of four research questions, formulated in Table 1.
Methods
A narrative review methodology was conducted to provide a broad overview of the current evidence on the relationship between COPD and VAP. The database search was the first step used to review the literature. The databases used were EMBASE and PubMed. The second element of the search was through internet sites, particularly the WHO site. The third element was to identify relevant articles from all the literature obtained.
The database search was conducted using a combination of the following Mesh Terms and keywords: chronic obstructive pulmonary disease, chronic obstructive airway disease, obstructive lung disease, chronic bronchitis; ventilator associated pneumonia; intensive care. Keywords were combined using the Boolean operators (AND, OR).
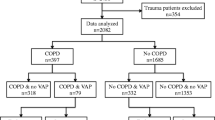
The articles which met the criteria for inclusion needed to be for adult population (> 18 years) and published in English between January 2005 and October 2018, plus prior references in the selected articles. Current recommendations from the Cochrane Foundation to conduct the literature search were followed. Review articles were not included. Further breakdowns of the search strategy are summarised in Fig. 1.
Is COPD a risk factor for VAP development?
The current literature on whether COPD is a risk factor for VAP development is controversial.
Data from the EU-VAP project demonstrated that VAP prevalence and incidence did not differ between patients with and without COPD [5]. The EU-VAP project was a prospective, observational cohort study developed in 27 ICUs from 9 different countries in Europe; it is the largest study examining the relationship of COPD and VAP from multiple European centres [5]. Excluding trauma patients, 397 out of 2082 patients had COPD (19.1%) [5]. Baseline characteristics and severity of illness at ICU admission did not differ between COPD patients with and without VAP development, and there was no significant difference in the median onset of VAP between the two groups [5]. The reported prevalence of VAP did not differ significantly between COPD and non-COPD ICU patients (18.6% vs. 18.2%, respectively, ns). Similarly, VAP incidence was not significantly different between patients with and without COPD, even when those with neurological major organ failure and CAP on admission were excluded (15.5 vs 17.5 per 1000 ventilation days at risk, ns) [5]. Therefore, the above study did not identify COPD as a risk factor for VAP development [5].
Similar results were obtained by Rodríguez et al. [24] in a prospective, observational, case-control study of 235 patients receiving MV in two multidisciplinary ICUs. Of these patients, 60 (25.5%) had a non-exacerbated COPD, while the remaining 175 (74.5%) were not diagnosed with COPD, representing the control group [24]. When premorbid pulmonary function tests were not available, clinical criteria, along with medical records with compatible physical findings and evidence of hyperinflation on chest radiograph, were used for COPD diagnosis [24]. The unadjusted analysis showed that the incidence of VAP in patients with COPD was 16.6% (10 patients), versus 36.0% (63 patients) in patients without COPD [24]. However, after adjustment of VAP episodes per 1000 MV days, no significant difference was noticed between those with COPD and those without (11.9/1000 vs 16.0/1000) [24]. Therefore, the study concluded that intubated patients with non-exacerbated COPD were not exposed to a higher risk of VAP, suggesting that VAP development was associated with extra days of mechanical ventilation rather than with COPD as a comorbidity [24]. Furthermore, the median VAP development in COPD patients was 7.5 days, and 4 days in patients without COPD [24]. No significant difference was seen in the development of VAP between survivors (13.2%) and non-survivors (22.7%) [24]. Patients with exacerbated COPD were not included in the study. Limitations of this study include imbalances in comorbidities and admission types between the cases and control patients as well as a lack of information regarding the cause of death [24]. In addition, spirometry data prior to ICU admission were only available for less than 20% of COPD patients, while the remaining cases were defined as COPD without using an objective criteria [24].
Contrary to the above, Tejerina et al. [25] conducted a retrospective analysis of a database from a prospective, multicentre international cohort from 20 countries of 2897 patients who received mechanical ventilation for > 12 h [25]. VAP developed in 439 (15%) patients and, in the multivariate analysis, COPD was identified as a risk factor for VAP development (OR 95% CI 3.9 (2.2–6.9), p < 0.001).
Along the same lines as the above studies were the results of a meta-analysis related to VAP in populations undergoing cardiac surgery, of which five studies [26,27,28,29,30] yielded similar results to the above [31]. The analysis of the 6416 patients recruited in these five studies shows that VAP occurred more frequently in patients with COPD (fixed effect model, 95% CI, 1.18, 2.01, p < 0.01). Chang et al. [32], Karatas et al. [33], Liu et al. [34] and Al-Dorzi et al. [35] also identified COPD as a risk factor for VAP development. Interestingly, Saied et al. [36] reported that the risk factors for VAP differ based on the time of onset, with COPD being associated with increased risk for developing late onset pneumonia.
On the other hand, Rinaudo et al. [37], in a prospective observational case-control study, analysed the role of COPD in 279 patients with ICU-acquired pneumonia (ICUAP), both with VAP (156) and with non-ventilator associated pneumonia (NV-ICUAP: 123). In contrast to other studies, subjects with absence of pulmonary function testing (PFT) were not included in the study [37]. Current and former smokers (≥ 10 pack-years) without a clinical diagnosis of COPD and without PFT were excluded to avoid misclassification of undiagnosed COPD in the non-COPD group, while those with forced spirometry without obstructive ventilator pattern were included in the non-COPD group [37]. COPD severity was assessed with the GOLD criteria [12]: 9 patients were stage I (13%), 24 stage II (34%), 30 stage III (42%) and 8 stage IV (11%) [37]. Interestingly, the prevalence of VAP development was lower in patients with COPD than those without COPD (42% [30/71] vs. 61% [126/208], respectively, p = 0.011) [37]. In this study, patients with COPD had a lower incidence of VAP, but a higher incidence of non-ventilator ICU-acquired pneumonia [37]. A strength of the study is the well-defined population of patients with and without COPD, whereas the lack of microbiological documentation in 41% of ICUAP cases represents one of the study’s limitations [37].
Finally, Gursel et al. [38], in a retrospective analysis of a prospective computerised database of a Turkish ICU, explored the role of co-existence of bronchiectasis with COPD and reported that VAP development was significantly higher in patients with COPD plus bronchiectasis vs. COPD alone (60% vs. 45%, p = 0.034).
Does COPD impact the outcome of patients with VAP?
COPD has an adverse impact on respiratory muscle function, which can be amplified during critical illness. Other factors such as older age and frequent use of corticosteroids may also be accountable for the high mortality rate reported in COPD patients developing VAP [11].
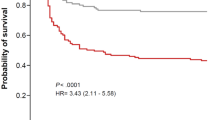
In the study of Rinaudo et al., patients’ outcome was monitored up to 90 days after pneumonia onset, and COPD was found to be independently associated with a decrease in survival in patients who had VAP [37]. The 90-day mortality rate was significantly higher in COPD patients with VAP (18 (62%) vs 45 (37%); p = 0.015) [37]. Consistent with the latter findings, a cumulative survival curve at 90 days was significantly different in VAP patients with COPD, with difference in mortality increasing even after discharge, suggesting delayed effects possibly due to hyperinflammation after pneumonia resolution. The authors did not analyse the impact that the severity of lung obstruction according to GOLD staging had on outcomes [37]. Notably, the association between COPD and worse survival was seen only in VAP patients, and COPD was not associated with worse survival in patients who developed non-ventilator ICUAP [37]. Additionally, the sequential organ failure assessment (SOFA score), previous use of corticosteroids and serum levels of IL-6 and IL-8 were found to be lower in COPD patients who had a non-ventilator ICUAP [37]. This might explain why COPD was associated with a higher mortality in VAP patients only, as these factors may have counterbalanced the results [37]. The multivariate analysis showed that COPD was independently associated with worse survival in VAP patients [37].
Similarly, Makris et al. [39] identified COPD as an independent risk factor for ICU mortality in patients with VAP. This single-centre, prospective, observational study included 215 patients with VAP; 65 (30%) of them had COPD (GOLD stage I 4 (7.8%), stage II 18 (35.2%), stage III 14 (27.4%), stage IV 15 (29.5%) [39]. Patients were classified as having COPD based on spirometry or prior diagnosis; for 21% of COPD patients, in which baseline spirometry could not be performed, the severity of COPD was assessed based on previous official medical records [39]. Only microbiologically confirmed cases of COPD were included in the study cohort [39]. COPD patients were found to have a mortality rate significantly higher than non-COPD patients (60% vs. 43%, p = 0.027) [39]. The mortality rate differed based on GOLD stages of severity and were 25%, 82%, 77.5%, and 66% for stages I, II, III, and IV, respectively [39]. In the multivariate analysis, after adjusting for potential confounding factors, such as comorbidities, advanced age, severity of VAP and severity of critical illness, COPD remained one of the risk factors for mortality (OR 2.58, 95% CI 1.33–5.02, p = 0.005), along with a higher simplified acute physiology score (SAPS II) at ICU admission and presence of shock on the day of VAP diagnosis [39]. No significant association was found between ICU stay and mechanical ventilation in less severe COPD patients (GOLD stage I–III); however, patients with advanced COPD (GOLD stage IV) were found to have significantly longer ICU stay and duration of mechanical ventilation compared to non-COPD patients [39]. In the sub-group of survivors, stage IV COPD patients had marginally longer mechanical ventilation duration (69 (24–103) vs. 26 (15–41), p = 0.005) and ICU stay (74 (37–113) vs. 34 (28–58), p = 0.07) compared to non-COPD patients [39]. Limitations of this study include the significant differences between COPD and non-COPD subgroups, although these were partially accounted for by the multivariate analysis adjustments, and the potential underestimation of COPD prevalence [39]. Prior COPD diagnosis or spirometry was compulsory for COPD definition; however, not all patients with COPD had been previously diagnosed [39]. Indeed, it is not uncommon for COPD to be firstly diagnosed during ICU admission based on clinical and radiological findings [39]. Therefore, current or former smokers with COPD might have been misclassified as non-COPD due to the absence of prior clinical diagnosis or PFTs [39].
Similarly, Lisboa et al. [40] conducted a prospective, observational cohort study in 3 Spanish ICUs on 441 patients with VAP in which COPD resulted being more common in VAP non-survivors than in survivors (27.6% vs. 16.9%, p < 0.05).
Lastly, a prospective study by Rello et al. recruited 129 consecutive episodes of VAP over 35 months [41]. Antecedent COPD was diagnosed using the standard criteria recommended by the American Thoracic Society [42]. In the univariate analysis, antecedent COPD was found to be significantly associated with attributable mortality in VAP patients [41]. However, when a step-forward logistic regression analysis was applied, the effects of antecedent COPD on VAP mortality were not statistically significant [41].
Does VAP development impact the outcome of COPD patients?
Most studies conclude that VAP development impacts the outcome of COPD patients in the ICU; however, some studies did not identify a correlation between VAP and worse outcomes.
According to a secondary analysis on the EU-VAP project, VAP leads to a longer duration of mechanical ventilation and hospital stay and is an independent risk factor for mortality in the ICU [5]. The development of VAP in COPD patients resulted in an increase of 17% in mortality rate compared to COPD patients that did not develop VAP (48.1% vs 31.1%, p = 0.005) [5]. Furthermore, the median duration of mechanical ventilation was reported to be 12 days longer (18 vs 6 days, p < 0.001), as well as the median length of ICU stay (23 vs 9 days, p < 0.001) [5]. VAP, along with SAPS II, were identified as independent predictors of mortality in ICU patients [5]. McCabe chronic disease status was assessed, but the severity of COPD based on GOLD staging was not recorded; therefore, we could not conclude whether there is a relationship between COPD severity and VAP or ICU outcome [5].
Similar results were obtained by Nseir et al. [43] in a single-centre, prospective, observational, case-control study conducted on a total of 1241 patients diagnosed with COPD, of which 77 (6%) developed VAP, with the vast majority being late-onset (71%). Length of ICU stay (26 ± 17 vs 15 ± 13, p < 0.001), duration of mechanical ventilation (24 ± 15 vs 13 ± 11, p < 0.001) and mortality rate (50 vs 22, p < 0.001) were all higher in COPD patients who developed VAP compared to control patients; VAP was identified as an independent risk factor for mortality in COPD patients in the ICU (64% vs 28%, p < 0.001) [43]. However, mortality rate, length of ICU stay and length of MV were all significantly lower in VAP patients receiving corticosteroids [43]. Limitations of this study include the fact that COPD severity was not recorded [43].
In agreement with the above findings are the results of a single-centre, prospective, observational, clinical study conducted by Badawy et al. [44]. Patients with an acute exacerbation of COPD and in need for MV for ≥ 48 h were included in the study. From 152 included patients, 92 developed VAP [44]. VAP was identified as a risk factor for morbidity and mortality in COPD patients: the risk of death in COPD patients with VAP was as high as 47.8%, compared to 30% in COPD patients without VAP [44]. VAP caused by multidrug-resistant organisms was particularly associated with an increased risk for mortality in ICU COPD patients, due to a higher risk of receiving the wrong antibiotic treatment [44]. COPD patients who developed VAP were also seen to have an ICU stay of 18.2 ± 8.8 (vs 7.4 ± 2.9 in COPD without VAP, P = 0.0001), a MV duration of 15 ± 8.07 (vs 4.23 ± 1.5 in COPD without VAP), and a clinical pulmonary infection score (CPIS) of 8.8 ± 1.7 (vs 4.21 ± 1.5 in COPD without VAP) [44]. Older age, late-onset VAP, re-intubation and prolonged use of antibiotics were identified as predictors of mortality in COPD patients who developed VAP [44].
In addition, in another retrospective single-centre study by Gursel [17] which included 86 COPD patients, a logistic regression analysis showed that VAP was an independent predictor for ICU stay > 10 days in patients with COPD. VAP was also identified as an independent predictor for MV > 7 days (OR 6; 95% CI 2–23, p = 0.011) and MV > 15 days (OR 14, 95% CI 3–66, p = 0.001), but it was not a risk factor for MV > 21 days [17].
Contrary to the results of the abovementioned studies, in the study by Rodriguez et al. [24], although COPD was overall an independent risk factor for mortality (HR 2.1, 95% CI 1.10–3.94), the mortality rate was not significantly different between patients with non-exacerbated COPD who developed VAP compared to those without VAP (50% vs. 34%, respectively).
Along the same lines as above were also the results of the study by Hadda et al. [45], conducted in patient with exacerbated COPD. This retrospective, single-centre study was conducted in an Indian hospital and included 186 ICU patients with an exacerbation of COPD; 82% (153) required intubation and mechanical ventilation, and of these, 23% (35) developed VAP (¾ were late-onset VAP) [45]. Neither in-hospital nor 28-day mortality rate of intubated COPD patients was significantly different between patients who developed VAP and those who did not (51% vs 53.4% and 48.6% vs 46.6%, respectively) [45]. Duration of mechanical ventilation (32 ± 10 vs 10 ± 2, p < 0.031) and ICU stay (53 ± 26 vs 18 ± 7, p < 0.001) were both longer in COPD patients with VAP [45]. The main strength of the study is that it is a homogenous cohort, while the main limitation is the retrospective study design, which makes it difficult to establish a cause–effect relationship [45].
Does COPD impact the aetiology of VAP?
COPD leads to physiological changes which predispose patients to infections. The loss of epithelium integrity and impairment of mucosal clearance lead to an increased risk for infection in patients with COPD, particularly from Gram-negative bacilli [46]. Whether antipseudomonal therapy should be part of empiric therapy, even in early onset pneumonia, is an important issue.
Koulenti et al. [5] observed that the prevalence of Pseudomonas aeruginosa VAP was 26.4% in COPD patients, vs 15.8% in patients without COPD. The prevalence of non-fermenting Gram-negative bacilli VAP was noticed to be 49.9% in COPD and 18.5% in non-COPD patients (p = 0.020), and it was even higher in COPD patients with an early onset-VAP (54.1% vs 20%, p < 0.001), which is particularly significant for the selection of empirical antibiotic therapy [5]. Similar results were obtained by Nseir et al. [43], who observed that P. aeruginosa (31%), A. baumannii (19%) and S. aureus (14%) were the most frequently isolated microorganisms in VAP patients with COPD. The prevalence of multidrug-resistant (MDR) bacteria in these patients was found to be 41% [43]. The most frequently isolated microorganisms by Makris et al. [39] were again P. aeruginosa (39%) and S. aureus (17%); a total of 136 MDR bacteria were isolated in 57% of the patients. In the same line were the results of the retrospective study by Gursel [17], who reported P. aeruginosa as the most commonly isolated pathogen. Moreover, in another study from the same author [38], the impact of co-existence of bronchiectasis with COPD was explored and P. aeruginosa was reported as being more frequent in COPD in the presence of bronchiectasis versus COPD alone [38]. However, the study was small and a larger study is needed to shed light on the impact of bronchiectasis/COPD combination on VAP aetiology [38].
Contrary to the above, a study by Badawy et al. [44] on microbiologically confirmed VAP in patients with exacerbated COPD, Gram-negatives (56%) were still the most frequent pathogens, but the most frequent isolate was Klebsiella spp. followed by E. coli, while MRSA was the most frequent Gram-positive pathogen.
Finally, Rello et al. [47], in a prospective, observational single-centre study that included 72 cases with microbiologically confirmed VAP out of 568 mechanically ventilated patients, reported that COPD was an independent risk factor for P. aeruginosa VAP (RR 29.9, 95% CI 4.86–184.53).
The summary of the main studies included in this review (since 2005) are presented in Table 2, with depicted differences in settings and definitions.
Conclusions
This manuscript critically and comprehensively reviews the current literature on the interaction between VAP and COPD, a topic that has important clinical implications for the decision making of ICU physicians. In subjects undergoing mechanical ventilation, there were studies that identified COPD as a risk factor for VAP development, as opposed to other studies where COPD was not observed to cause a higher incidence of VAP. The literature on whether antecedent COPD is a risk factor for worse outcomes in patients who develop VAP is also controversial. However, most studies agree that VAP development increases morbidity and mortality of ICU patients with COPD. Regarding VAP aetiology, the most common causative agents of VAP in COPD patients were Gram-negative bacilli, with Pseudomonas aeruginosa being the most prevalent.
The identified controversy on the findings may be a result of heterogeneity in definitions used for VAP and COPD diagnosis, i.e. for VAP, the need or not of microbiological confirmation, sampling/culture methods (e.g. bronchoscopic vs. non-bronchoscopic, qualitative vs. quantitative cultures); for COPD, the need for pulmonary function tests vs. diagnosis based on history/clinical findings/imaging. Moreover, differences in the cohorts, such as case-mix, demographics, comorbidities, severity of illness and management practices might have contributed to the controversial findings as well.
Methodological aspects that need improvement have been detected and priorities for further research in the field have been elucidated. Unfortunately, only a few references for each research question used logistic regression models, i.e. COPD impact on VAP incidence [25, 32, 34] and outcomes [37, 39,40,41], and VAP impact on COPD outcomes [5, 17, 38, 43, 44]. Identification of COPD subgroups that would benefit the most from more stringent VAP prevention measures could lead to a decrease in VAP development and related healthcare costs. On the other hand, the elucidation of COPD’s impact on the aetiology of early-onset VAP could lead to a change in the current guidelines for the empirical management of VAP for patient with underlying COPD or for specific subgroup of COPD patients. Further studies with stratification of severity based on the GOLD staging are warranted.
Change history
28 February 2019
Unfortunately, the Acknowledgements section was not included in the original version of the article. The said section is given here.
References
American Thoracic Society (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416. https://doi.org/10.1164/rccm.200405-644ST
Kalil AC, Metersky ML, Klompas M et al (2016) Management of Adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. https://doi.org/10.1093/cid/ciw353
Koulenti D, Lisboa T, Brun-Buisson C et al (2009) Spectrum of practice in the diagnosis of nosocomial pneumonia in patients requiring mechanical ventilation in European intensive care units. Crit Care Med 37:2360–2369. https://doi.org/10.1097/CCM.0b013e3181a037ac
Koulenti D, Tsigou E, Rello J (2017) Nosocomial pneumonia in 27 ICUs in Europe: perspectives from the EU-VAP/CAP study. Eur J Clin Microbiol Infect Dis 36:1999–2006. https://doi.org/10.1007/s10096-016-2703-z
Koulenti D, Blot S, Dulhunty JM et al (2015) COPD patients with ventilator-associated pneumonia: implications for management. Eur J Clin Microbiol Infect Dis 34:2403–2411. https://doi.org/10.1007/s10096-015-2495-6
Rello J, Lisboa T, Koulenti D (2014) Respiratory infections in patients undergoing mechanical ventilation. Lancet Respir Med 2:764–774. https://doi.org/10.1016/S2213-2600(14)70171-7
Warren DK, Shukla SJ, Olsen MA et al (2003) Outcome and attributable cost of ventilator-associated pneumonia among intensive care unit patients in a suburban medical center. Crit Care Med 31:1312–1317. https://doi.org/10.1097/01.CCM.0000063087.93157.06
Kollef MH, Hamilton CW, Ernst FR (2012) Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol 33:250–256. https://doi.org/10.1086/664049
Melsen WG, Rovers MM, Groenwold RH et al (2013) Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis 13:665–671. https://doi.org/10.1016/S1473-3099(13)70081-1
Vandana Kalwaje E, Rello J (2018) Management of ventilator-associated pneumonia: need for a personalized approach. Expert Rev Anti-infective Ther 16:641–653. https://doi.org/10.1080/14787210.2018.1500899
Celli BR (2010) Predictors of mortality in COPD. Respir Med 104:773–779. https://doi.org/10.1016/j.rmed.2009.12.017
Rabe KF, Hurd S, Anzueto A et al (2007) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 176:532–555. https://doi.org/10.1164/rccm.200703-456SO
World Health Organisation World Health Statistics 2008. 2008 ; Available from: http://www.who.int/whosis/whostat/EN_WHS08_Full.pdf?ua=1. [accessed 2018 December 18]
Akkutuk E, Karakurt Z, Salturk C et al (2014) How do COPD comorbidities affect ICU outcomes? Int J Chron Obstructive Pulm Dis:1187. https://doi.org/10.2147/COPD.S70257
Chastre J, Fagon J-Y (2002) Ventilator-associated pneumonia. Am J Respir Crit Care Med 165:867–903. https://doi.org/10.1164/ajrccm.165.7.2105078
Restrepo MI, Sibila O, Anzueto A (2018) Pneumonia in patients with chronic obstructive pulmonary disease. Tuberc Respir Dis 81:187. https://doi.org/10.4046/trd.2018.0030
Gursel G (2005) Determinants of the length of mechanical ventilation in patients with COPD in the intensive care unit. Respiration 72:61–67. https://doi.org/10.1159/000083402
Schmidt M, Demoule A, Deslandes-Boutmy E et al (2014) Intensive care unit admission in chronic obstructive pulmonary disease: patient information and the physician’s decision-making process. Crit Care 18:R115. https://doi.org/10.1186/cc13906
Funk G-C, Bauer P, Burghuber OC et al (2013) Prevalence and prognosis of COPD in critically ill patients between 1998 and 2008. Eur Respir J 41:792–799. https://doi.org/10.1183/09031936.00226411
Crim C, Calverley PMA, Anderson JA et al (2009) Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J 34:641–647. https://doi.org/10.1183/09031936.00193908
Ernst P, Gonzalez AV, Brassard P, Suissa S (2007) Inhaled corticosteroid use in chronic obstructive pulmonary disease and the risk of hospitalization for pneumonia. Am J Respir Crit Care Med 176:162–166. https://doi.org/10.1164/rccm.200611-1630OC
Myles PR, McKEEVER TM, Pogson Z et al (2009) The incidence of pneumonia using data from a computerized general practice database. Epidemiol Infect 137:709. https://doi.org/10.1017/S0950268808001428
Vinogradova Y, Hippisley-Cox J, Coupland C (2009) Identification of new risk factors for pneumonia: population-based case-control study. Br J Gen Pract 59:e329–e338. https://doi.org/10.3399/bjgp09X472629
Rodríguez A, Lisboa T, Solé-Violán J et al (2011) Impact of nonexacerbated COPD on mortality in critically ill patients. Chest 139:1354–1360. https://doi.org/10.1378/chest.10-2439
Tejerina E, Frutos-Vivar F, Restrepo MI et al (2006) Incidence, risk factors, and outcome of ventilator-associated pneumonia. J Crit Care 21:56–65. https://doi.org/10.1016/j.jcrc.2005.08.005
Hortal J, Giannella M, Pérez MJ et al (2009) Incidence and risk factors for ventilator-associated pneumonia after major heart surgery. Intensive Care Med 35:1518–1525. https://doi.org/10.1007/s00134-009-1523-3
Pawar M, Mehta Y, Khurana P et al (2003) Ventilator-associated pneumonia: incidence, risk factors, outcome, and microbiology. J Cardiothorac Vasc Anesth 17:22–28. https://doi.org/10.1053/jcan.2003.4
Sheng W, Xing Q, Hou W et al (2014) Independent risk factors for ventilator-associated pneumonia after cardiac surgery. J Investig Surg 27:256–261. https://doi.org/10.3109/08941939.2014.892652
Tamayo E, Álvarez FJ, Martínez-Rafael B et al (2012) Ventilator-associated pneumonia is an important risk factor for mortality after major cardiac surgery. J Crit Care 27:18–25. https://doi.org/10.1016/j.jcrc.2011.03.008
Torres A, Aznar R, Gatell JM et al (1990) Incidence, risk, and prognosis factors of nosocomial pneumonia in mechanically ventilated patients. Am Rev Respir Dis 142:523–528. https://doi.org/10.1164/ajrccm/142.3.523
He S, Chen B, Li W et al (2014) Ventilator-associated pneumonia after cardiac surgery: a meta-analysis and systematic review. J Thorac Cardiovasc Surg 148:3148–3155.e5. https://doi.org/10.1016/j.jtcvs.2014.07.107
Chang L, Dong Y, Zhou P (2017) Investigation on risk factors of ventilator-associated pneumonia in acute cerebral hemorrhage patients in intensive care unit. Can Respir J 2017:1–4. https://doi.org/10.1155/2017/7272080
Karataş M, Saylan S, Kostakoğlu U, Yılmaz G (2016) An assessment of ventilator-associated pneumonias and risk factors identified in the intensive care unit. Pak J Med Sci 32. https://doi.org/10.12669/pjms.324.10381
Liu Y, Di Y, Fu S (2017) Risk factors for ventilator-associated pneumonia among patients undergoing major oncological surgery for head and neck cancer. Frontiers of Medicine 11:239–246. https://doi.org/10.1007/s11684-017-0509-8
Al-Dorzi HM, El-Saed A, Rishu AH et al (2012) The results of a 6-year epidemiologic surveillance for ventilator-associated pneumonia at a tertiary care intensive care unit in Saudi Arabia. Am J Infect Control 40:794–799. https://doi.org/10.1016/j.ajic.2011.10.004
Ibn Saied W, Souweine B, Garrouste-Orgeas M et al (2017) Respective impact of implementation of prevention strategies, colonization with multiresistant bacteria and antimicrobial use on the risk of early- and late-onset VAP: an analysis of the OUTCOMEREA network. PLoS One 12:e0187791. https://doi.org/10.1371/journal.pone.0187791
Rinaudo M, Ferrer M, Terraneo S et al (2015) Impact of COPD in the outcome of ICU-acquired pneumonia with and without previous intubation. Chest 147:1530–1538. https://doi.org/10.1378/chest.14-2005
Gursel G (2006) Does coexistence with bronchiectasis influence intensive care unit outcome in patients with chronic obstructive pulmonary disease? Heart Lung 35:58–65. https://doi.org/10.1016/j.hrtlng.2005.04.003
Makris D, Desrousseaux B, Zakynthinos E et al (2011) The impact of COPD on ICU mortality in patients with ventilator-associated pneumonia. Respir Med 105:1022–1029. https://doi.org/10.1016/j.rmed.2011.03.001
Lisboa T, Diaz E, Sa-Borges M et al (2008) The ventilator-associated pneumonia PIRO score. Chest 134:1208–1216. https://doi.org/10.1378/chest.08-1106
Rello J, Ausino V, Ricart M et al (1993) Impact of previous antimicrobial therapy on the etiology and outcome of ventilator-associated pneumonia. Chest 104:1230–1235. https://doi.org/10.1378/chest.104.4.1230
American Thoracic Society (1987) Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis 136:225–244. https://doi.org/10.1164/ajrccm/136.1.225
Nseir S, Di Pompeo C, Soubrier S et al (2005) Impact of ventilator-associated pneumonia on outcome in patients with COPD. Chest 128:1650–1656. https://doi.org/10.1378/chest.128.3.1650
Badawy MS, Omar HM, Mohamdien HA et al (2015) Evaluation of risk factors of ventilator associated pneumonia on outcome of acute exacerbation of chronic obstructive pulmonary disease. Egypt J Chest Dis Tuberc 64:799–803. https://doi.org/10.1016/j.ejcdt.2015.06.005
Hadda V, Dubey G, Nallan R et al (2014) Impact of ventilator associated pneumonia on outcome in patients with chronic obstructive pulmonary disease exacerbation. Lung India 31:4. https://doi.org/10.4103/0970-2113.125886
Talon D, Mulin B, Rouget C et al (1998) Risks and routes for ventilator-associated pneumonia with Pseudomonas aeruginosa. Am J Respir Crit Care Med 157:978–984. https://doi.org/10.1164/ajrccm.157.3.9702096
Rello J, Ausina V, Ricart M et al (1994) Risk factors for infection byPseudomonas aeruginosa in patients with ventilator-associated pneumonia. Intensive Care Med 20:193–198. https://doi.org/10.1007/BF01704699
Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36:309–332. https://doi.org/10.1016/j.ajic.2008.03.002
Celli BR, MacNee W, Agusti A et al (2004) Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 23:932–946. https://doi.org/10.1183/09031936.04.00014304
Heyland DK, Cook DJ, Griffith L et al (1999) The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. Am J Respir Crit Care Med 159:1249–1256. https://doi.org/10.1164/ajrccm.159.4.9807050
CDC (2003) NNIS criteria for determining nosocomial pneumonia
Pugin J, Auckenthaler R, Mili N et al (1991) Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis 143:1121–1129. https://doi.org/10.1164/ajrccm/143.5_Pt_1.1121
Yangco B (1989) CDC definitions for nosocomial infections. Am J Infect Control 17:42–43. https://doi.org/10.1016/S0196-6553(89)80013-6
American Thoracic Society (1995) Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 152(suppl):S77–S121
Funding
Supported in part by Observership Grant Programme (ESCMID, Basel, Switzerland) and PCI Pneumonia - Centro de Investigacion Biomedica en Red en Enfermedades Respiratorias (CIBERES), Madrid, Spain.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Koulenti, D., Parisella, F.R., Xu, E. et al. The relationship between ventilator-associated pneumonia and chronic obstructive pulmonary disease: what is the current evidence?. Eur J Clin Microbiol Infect Dis 38, 637–647 (2019). https://doi.org/10.1007/s10096-019-03486-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-019-03486-2