Abstract
We report on intensive care nosocomial pneumonia (NP) in Europe through a review of EU-VAP/CAP manuscripts: a prospective observational study, enrolling patients from 27 ICUs in nine European countries. From 2,436 eligible ICU patients, 827 cases presented NP, with 18.3 episodes of VAP per 1000 ventilator-days. Most common findings were worsening oxygenation, purulent respiratory secretions and temperature increase. At least three criteria from Clinical Pulmonary Infection score (CPIS) were present in 77.9 % of episodes, but only 0.2 % met six CPIS criteria. Diagnosis was confirmed mainly noninvasively (74.8 %), with half qualitative and quantitative cultures. The dominant isolate was S. aureus in Spain, France, Belgium and Ireland, P. aeruginosa in Italy and Portugal, Acinetobacter in Greece and Turkey, but Escherichia coli in Germany. NP resulted in 6 % higher mortality, longer ICU stay and duration of mechanical ventilation (12 and 10 days). COPD and age ≥45 years were not associated with higher VAP incidence but did correlate with increased mortality. Trauma had higher VAP incidence but lower mortality. Bacteremia (led by MRSA and Acinetobacter baumannii) was documented in 14.6 %, being associated with extra ICU stay and mortality. Vasopressors and ICUs with above 25 % prevalence of Potential Resistant Organisms (PRM) were independently associated with PRM, being documented in 50.7 % of patients with early-onset VAP without known risk factors. Most patients initially received combination therapy. Delay in appropriate antimicrobial choice significantly increased mortality, and LOS in survivors was six days longer (p < 0.05). In conclusion, NP management in Europe presents local differences and major shifts when compared to reports from North America, outcomes of randomized trials and general guidelines.
Similar content being viewed by others
Introduction
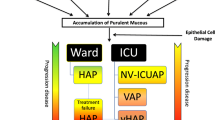
Incidence of ventilator-associated pneumonia (VAP) in the last decade has ranged between 1.9 and 3.8 per 1000 ventilator-days in the United States but exceeds 18 cases per 1000 ventilator-days in Europe [1–10]. Indeed, nosocomial pneumonia (NP) in the ICU also includes severe hospital-acquired pneumonia (HAP) [4–6]. Because the presence of an artificial airway increases the incidence by 6–20-fold [1, 5–8], VAP represents approximately 80 % of NP [1]. In this article, we compile findings on NP from the EU-VAP/CAP study to give a comprehensive perspective from Europe.
Methodology used in EU-VAP/CAP
Twenty-seven ICUs were involved from Belgium, France, Germany, Greece, Italy, Ireland, Portugal, Spain, and Turkey (Appendix 1). The study was conducted in 2006 and data collection lasted for approximately 6–12 months, depending on the size and type of the participating ICU. EU-VAP/CAP was a prospective, observational study that aimed to elucidate the incidence, the clinical presentation, the methods of diagnosis, and the outcome of nosocomial pneumonia in ventilated patients [11]. In subsequent secondary analyses further issues were clarified, namely, the special characteristics of VAP in trauma patients [12], in chronic obstructive pulmonary disease (COPD) patients [13], in elderly patients [14], the effect of pneumonia-associated bacteremia on outcomes [15], the incidence of potentially resistant isolates (PRM) in NP [16], the factors guiding choices of empirical antibiotic therapy [17], and the influence of nurse-to-patient ratio on the outcome [18].
Eligible patients were all those admitted to ICU with a diagnosis of pneumonia and also all ICU patients requiring invasive mechanical ventilation for ≥48 hrs, irrespective of the admission diagnosis. The target was the collection of data on 100 consecutive admissions in each ICU. Patients with community-acquired pneumonia (CAP) were not included in the present review.
HAP was defined as a lung infection presenting in non-intubated patients 48 hours or more after hospital admission, which was not already incubating at the time of such hospital admission [1]. VAP was defined as pneumonia arising 48 hours or more after endotracheal intubation with no evidence of pneumonia at the time of intubation. VAP was also defined after the diagnosis of a new pulmonary infection if the initial admission to ICU was for pneumonia [1]. Early-onset VAP was defined as VAP developing 2–4 days after intubation, while late-onset was defined as VAP developing ≥5 days after intubation [1]. Very early-onset VAP was defined as pneumonia developing within 48 hours after intubation [19]. Patients were classified as “middle aged” (45–64 years old), “old” (65–74 years old), and “very old” (≥75 years old) [14]. Trauma was defined as the presence of injury in more than one body area or system, or the presence of major cranial trauma alone [12]. COPD was defined as a pre-existing disease state characterized by the presence of airflow limitation due to chronic bronchitis or emphysema [13].
Microbiologically confirmed VAP was defined as VAP with a microorganism isolated from respiratory samples or blood in a patient with suspicion of pneumonia and characterized as definite etiology of VAP according to clinical judgment and interpretation of specimen culture results [1]. According to the 2005 American Thoracic Society/Infectious Diseases Society of America (ATS/IDSA) guidelines for HAP, VAP and HCAP, patients were aggregated into two groups: group 1, early-onset pneumonia without risk factors for PRM infection; and group 2, early-onset pneumonia with risk factors for PRM infection (who have received prior antibiotics or who have had prior hospitalization within the past 90 days and immunosuppressive disease and/or therapy) or late-onset pneumonia [1]. Participating ICUs with a prevalence of PRM greater than 25 % were considered high risk for PRM acquisition [16]. The initial empiric therapy was judged as appropriate when the causative organism was susceptible to one or more of the prescribed antibiotics with in vitro testing. Bacteremia was defined as at least one positive blood culture not related to another source of infection and at least one positive respiratory sample culture obtained within 48 hours of each other if two, with the same microorganism isolated in blood and respiratory samples. Septic shock followed published definitions from the 1992 American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference on sepsis and organ failure [20]. Organ dysfunction was defined according to SOFA score definitions [21]. Prevalence of pneumonia was expressed per 1000 patients mechanically ventilated for at least 48 hours, and occurrence rate of pneumonia was expressed per 1000 ventilator-days. Details on statistical analyses can be found elsewhere [11–18].
Overview of results obtained during EU-VAP/CAP
From the 2587 recruited patients, 2436 eligible patients were evaluated; 1089 were admitted with pneumonia or developed pneumonia during their ICU stay (Fig. 1). CAP was responsible for 262 (24.1 %) and NP for 827 (75.9 %) cases. Among NP, HAP occurred in 224 (20.6 %), VAP in 465 (42.7 %) and VE-VAP in 138 (12.7 %) cases. Late-onset VAP accounted for 58.5 % of VAP cases (Fig. 1).
Incidence and clinical presentation of nosocomial pneumonia [11]
The overall incidence of VAP was 18.3 episodes per 1000 ventilator-days, with the diagnosis established within the first 10 days of ICU admission in 89.1 % of cases. The prevalence was 8.1 % for HAP, 18.0 % for VAP, and 5.5 % for VE-VAP. The most common findings of nosocomial pneumonia were worsening oxygenation, purulent or changing respiratory secretions and new temperature elevation. At least three criteria included in the clinical pulmonary infection score (CPIS) were present in 77.9 % of patients, but only 0.2 % of patients met six CPIS criteria.
Diagnostic materials [11]
The principal diagnostic material of nosocomial pneumonia were noninvasive respiratory specimens, performed in 74.8 % of cases (ranging 0–100 %). Tracheal aspirates were actually the only respiratory specimen collected in 51.8 % of cases and the only procedure performed for etiological diagnosis in 14.8 %. Qualitative and quantitative cultures of bronchial aspirates were performed in a quite similar fashion (46.2 and 56.3 %, respectively). Bronchoscopy was used in 23.3 % of cases and it was the only procedure used in 8.1 %. Bronchoscopy performance was associated with worsening oxygenation (OR 2.03). Bronchoscopic lavage (BAL) was performed in 18.4 %, bronchoscopic protected specimen brush (PSB) in 6.5 % with BAL fluid cytology in 3.5 % of cases, mainly from university/university-affiliated ICUs. Blind mini-BAL was used in 8.6 %, and blind PSB in 11 % of cases. Blood cultures were performed in 69.2 %. Serology on serum and pleural fluid cultures were performed in 4.8 and 5.8 %, respectively.
Microbiology [11]
In 69.5 % of suspected NP, a pathogen was documented. There was a high variability in microbiologic documentation between the participating ICUs, ranging from 41.2 to 94.9 %. The most common isolates were Enterobacteriaceae, Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter baumannii. Polymicrobial infection was documented in 32.2 %. Early-onset VAP compared with late-onset VAP showed a greater (p < 0.05) proportion of methicillin-susceptible S. aureus (27.6 vs. 11.4), Streptococcus pneumoniae (9.0 vs. 2.4), and Haemophilus influenzae/Moraxella catharralis (13.8 vs. 3.8) and lower Acinetobacter baumannii (11.0 vs. 26.5). A trend for a lower proportion of P. aeruginosa in early onset-VAP was also found (17.9 vs. 26.1, p = 0.09). The dominant isolate for Spain, France, Belgium and Ireland was S. aureus, for Italy and Portugal P. aeruginosa, for Greece and Turkey Acinetobacter and for Germany Escherichia coli.
Outcomes [11]
The overall ICU mortality rate was 34.4 %. Nosocomial pneumonia was estimated to be directly related to death in 19.6 % of patients who died and contributing to death in 43.9 %. However, patients with NP had only 6 % higher mortality rates compared to patients without pneumonia (37.7 % vs.31.6 %, p < 0.05). ICU patients who survived with NP had a longer ICU stay (25.6 ± 21.0 vs. 13.4 ± 13.0 days, p < 0.05) and a longer length of mechanical ventilation (18.9 ± 19.3 vs. 8.6 ± 9.4 days, p < 0.05) than survivors without pneumonia.
VAP in special populations
In secondary analyses, VAP was studied in specific patient populations, i.e. trauma, COPD and older patients, because based on published reports VAP presents distinct etiology, clinical characteristics and outcome in these sub-groups [22–28].
Trauma [12]
Overall, 36.5 % of trauma patients developed VAP. Trauma patients were younger and more frequently men, had a lower SAPS II score and had less comorbidities compared to the rest of the cohort. Most underwent multiple trauma (47.7 %), followed by head trauma (36.7 %). Though younger and healthier, trauma patients had a higher risk for VAP in the entire cohort (odds ratio [OR] = 2.89). Compared to non-trauma, trauma patients had no difference in septic shock, rate of bacteremia and prior antibiotics exposure.
Enterobacteriaceae were the most prevalent isolates in trauma (29.0 %), followed by Acinetobacter baumannii (22.9 %) and methicillin-susceptible Staphylococcus aureus (16.7 %). Comparing early and late VAP, Enterobacteriaceae spp. predominated in early VAP and Acinetobacter baumannii in late VAP.
ICU mortality in trauma was 17.2 %, lower than the one observed in the whole cohort, being maintained after controlling for potential confounders. After pneumonia onset, median length of ICU stay and duration on mechanical ventilation (16 and 10.5 days) were not longer in trauma patients who developed VAP.
COPD [13]
After excluding trauma, 2082 patients were assessed with 397 having COPD, of whom 79 developed VAP (19.8 %). VAP incidence was 18.2/1000 ventilator days at risk, with no significant difference, when patients with CAP and with neurological failure on admission were excluded. The median onset of VAP in patients with COPD was 6 (4–15) days. Compared to the initial cohort, VAP-COPD patients were older and with more comorbidities. However, they did not differ from non VAP-COPD patients in terms of demographics, clinical characteristics, comorbidities and severity-of-illness at admission. COPD patients who developed VAP showed a higher prevalence of hypothermia and pleural effusion compared to non-COPD patients, but no other difference was noted in physical and laboratory characteristics. Septic shock at VAP onset did not differ. Enterobacteriaceae was the most frequent pathogen in COPD patients, followed by Pseudomonas aeruginosa. Compared to patients without COPD the prevalence of Pseudomonas aeruginosa VAP (overall and with early onset) was higher (26.4 vs. 15.8 %, respectively; p = 0.037). Overall, the prevalence of non-fermenting Gram negative bacilli was higher in COPD patients (p = 0.056), especially in those with early-onset VAP (54.1 vs. 20.0 % in patients with and without COPD, respectively; p < 0.001).
COPD patients who developed VAP and survived had a longer ICU stay (median 13.5 days) and a longer duration of mechanical ventilation (median 12 days), compared to COPD patients that did not develop VAP. VAP-COPD patients had a higher mortality than patients in the overall cohort and also than COPD patients without VAP (48.1 vs. 31.1 %, p = 0.005). Using logistic regression analysis VAP and SAPS II score proved to be independent predictors of ICU mortality in patients with COPD.
Elderly patients [14]
After excluding trauma, 1735 patients aged ≥ 45 years were included in the analysis. The middle-aged group included 670 persons, the old group included 549 persons, and the very old group included 516 persons. Old and very old patients had a higher frequency of chronic diseases (chronic heart failure, chronic respiratory disease, chronic renal failure, diabetes, and non-metastatic cancer), but cirrhosis, solid organ transplantation, and alcoholism were more common among middle-aged patients.
The prevalence of VAP in the total cohort (patients aged ≥ 45 years) was 14.4 VAP/1000 ventilation days at risk. No important differences in occurrence rate of VAP were noted between the age groups (ranging from 14.1 to 18.9 % over the age groups), a finding that was also verified with logistic regression analysis. The only variable recognized as an independent risk factor for VAP was length of mechanical ventilation. The clinical picture of VAP did not differ between the age groups, except for new temperature rise which was less frequently observed in very old patients. In patients ≥65 years, Enterobacteriaceae, particularly Escherichia coli and Klebsiella pneumoniae, were the most frequent pathogens, followed by Pseudomonas aeruginosa, MRSA and Acinetobacter baumannii. Among very old subjects the predominance of Enterobacteriaceae was even more pronounced. Elderly patients had a higher mortality, which was 35 % in middle-aged patients versus 51 % in old and very old patients. Older age as an independent risk factor for death was confirmed in logistic regression analysis. In this analysis, being old or very old resulted in an identical risk for death. The three age groups had no significant difference in terms of the length of ICU stay and of mechanical ventilation.
The impact of bacteremia in the outcome of nosocomial pneumonia [15]
From the 689 cases of NP (465 VAP and 224 HAP) blood cultures were extracted in 479 (69.5 %) and were positive in 70 (14.6 %) patients. Bacteremic NP patients did not differ substantially from non-bacteremic NP patients, apart from the fact that they had a higher SAPS II score and they were more frequently medical patients. No difference was depicted in comorbidities. The diagnosis of VAP was set in a later time after ICU admission in bacteremic patients (7.3 ± 14.1 days vs. 4.9 ± 5.8 days, P = 0.02). Enterobacteriaceae were isolated in 29.7 % (especially Klebsiella and Escherichia coli), but MRSA was the single pathogen most frequently identified (22.6 %), followed by Acinetobacter baumannii (17.9 %).
Backward logistic regression analysis highlighted non-trauma cause of admission, MRSA, A. baumannii and duration of mechanical ventilation as independent risk factors for the development of bacteremia. ICU mortality was higher in bacteremic patients compared to non-bacteremic (57.1 vs. 33 %, p < 0.001). Actually, bacteremia proved to be an independent risk factor for mortality in backward logistic regression analysis (OR for death: 2.01, p = 0.008) after adjustment for severity-of-illness. The ICU length of stay was longer (28.5 ± 30.6 vs. 20.5 ± 17.1 days, p = 0.03) in bacteremic patients, whereas duration of mechanical ventilation had no significant difference.
Prediction of the prevalence of PRM in nosocomial pneumonia [16]
Only patients with an established definitive microbiologic diagnosis of NP (HAP/VAP) and antibiotic susceptibility results were included in this subgroup analysis. From the 485 patients studied, 152 (31.3 %) were allocated to group 1 with early-onset pneumonia and no risk factors for PRM acquisition, whereas 333 (68.7 %) were classified into group 2 with early-onset pneumonia with risk factors for PRM or late-onset pneumonia. Among the 152 patients from group 1 without risk factors for PRM, 77 patients (50.7 %) proved to have PRM. In 199 patients from group 2 (59.8 %), PRM were identified as the causative pathogens. In both groups Pseudomonas aeruginosa was the most frequently isolated pathogen, followed by MRSA and Acinetobacter baumannii (similar percentages), whereas Stenotrophomonas maltophilia was implicated in less than 5 % of cases.
According to a logistic regression analysis, the presence of severe sepsis/septic shock (OR 3.7, p < 0.01) and pneumonia which developed in centers with more than a 25 % prevalence of PRM (OR 11.3, p < 0.01) were independently associated with PRM causing pneumonia. Antibiotic prescription was in accordance with the 2005 ATS/IDSA guidelines in 46.4 % of the patients, with the percentage being even higher in group 1 patients.
ICU mortality was 39.4 % in the whole population. Group 1 patients presented a higher mortality than group 2 patients (42.6 vs. 32.2 %, p = 0.03), as did patients with PRM (47.5 vs.28.7 %, p < 0.01). PMR patients also received more inappropriate antimicrobial therapy (29.6 vs. 11.5 %, p < 0.01) than patients without PRM acquisition. Initial appropriate antibiotic therapy resulted in lower mortality rates compared to inappropriate therapy (35.4 vs. 48.1 %, p = 0.01).
Choice of empirical therapy in nosocomial pneumonia [17]
The main factors affecting prescription, apart from previous hospitalization and previous antibiotic use, that are well-known contributing factors, were admission category, hospital ecology and length of stay more than 5 days. Specifically, trauma patients were more likely to receive non-anti-Pseudomonas cephalosporins and surgical patients were less likely to receive aminoglycosides, and a trend towards MRSA agents was also noted in surgical patients. In ICUs with prevalence of A. baumannii >10 % the choice of empirical therapy was in favor of carbapenems and colistin (OR 3.50 and OR 115.71, respectively). Length of stay > 5 days was also associated with broader spectrum antibiotics, such as colistin (OR 5.25) and anti-MRSA agents (OR 1.43).
More than 30 antibiotic regimens for the initial treatment of VAP were identified. The 32.2 % of patients received one antibiotic, 36.8 % received two and 30.9 % received three or more antibiotics. The most prevalent agents were carbapenems (18.5 %), piperacillin/tazobactam (13.1 %) and fluoroquinolones (9.9 %). For five of the participating countries carbapenems were the first choice against VAP, in France cephalosporins were chosen more often, whereas in Belgium and Germany piperacillin/tazobactam was the agent of choice. Anti-MRSA antibiotics were prescribed as follows: vancomycin 17.5 %, linezolid 12.7 % and teicoplanin 8.2 % of episodes. Combination therapy was chosen for 67.7 % of patients, without differences between early versus late-onset VAP. Fluoroquinolones and aminoglycosides were included in the regimen in 26.7 and 23.2 % of cases, respectively. Anti-Pseudomonas agents were prescribed in 52.5 and 63.6 % of patients with early- and late-onset VAP, respectively. SAPS II score correlated with empirical antibiotic regimen and higher values corresponded to broader spectrum regimens. In HAP, similarly to VAP, more than 30 antimicrobial regimens were used for empiric therapy, and more than two third of patients received more than one antibiotic. Carbapenems were the first antibiotic of choice, followed by piperacillin/tazobactam, non-pseudomonas cephalosporins and cefepime/ceftazidime. Combination therapy was selected for 70.6 % of patients and included fluoroquinolones and aminoglycosides in percentages of 34.2 and 21.5 %, respectively.
Overall mortality was 35.7 %. In patients who received appropriate initial anti-microbial therapy, mortality was lower compared to patients who received inappropriate therapy (35.1 % versus 48.1 %; p = 0.01). LOS was six days lower (p < 0.05) in patients who received appropriate therapy (26.3 ± 19.8 versus 32.8 ± 29.4 days), and there was also a trend (p = 0.08) for 5 less days on mechanical ventilation (19.7 ± 18.7 versus 25.1 ± 28.7 days).
How the patient-to-nurse ratio affects the risk for VAP [18]
This analysis included 1,658 patients who had received mechanical ventilation for at least 48 hours, from 21 ICUs which have provided data on the nurse-to-patient ratio. In ten ICUs the patient-to-nurse ratio was 2/1, in ten the ratio was 2.5–3/1, and in one the ratio was 1/1. In ICUs with a patient-to-nurse ratio >1 there were more medical and trauma patients, the ICU length of stay and the duration of mechanical ventilation were longer, but the SAPS II score was lower compared to ICUs with 1/1 ratio. VAP rates in units with patient to nurse ratios of 1 to 1, 2 to 1, 2.5 to 1, and 3 to 1 were 9.3, 25.7, 18.7, and 24.2 %, respectively (P = 0.003); after correction for confounding factors the difference no longer existed. Instead, other factors such as trauma as the cause of admission, SAPS II score and number of days at risk (number of days of mechanical ventilation before the onset of VAP) were VAP determinants.
Global discussion of the overall results obtained during EU-VAP/CAP
EU-VAP/CAP investigators published on and highlighted many controversial aspects of nosocomial pneumonia affecting critically ill patients [11–18]. The present review provides a compilation of these sub-studies giving a global perspective of the disease in Europe [11–18]. The prevalence of HAP in the European ICUs was lower than Oceania, highlighting differences in health-care system organization and case mix [29]. Etiology, diagnostic techniques and empirical treatment patterns of VAP on the other hand, differed to that reported from North American ICUs [30].
Extraction of tracheal aspirates was used in 74.8 % of patients to document the diagnosis, and this contrasted with the myth, based on research studies, that bronchoscopy was the standard of care in Europe. The most common isolates in declining order were Enterobacteriaceae, Staphylococcus aureus, Pseudomonas aeruginosa, and Acinetobacter baumannii. The dominant isolates differed between the participating countries. Our findings support the interest of taking blood cultures, despite low positivity rates. Bacteremia resulted in higher ICU mortality and in longer ICU stay. In the EUROBACT study respiratory source of bacteremia was associated with a 1.5-fold increase in the 28-day mortality [31, 32].
Trauma patients had a higher risk for VAP acquisition compared to the whole cohort, even though they were younger and with less comorbidity. The incidence of VAP in COPD patients was similar to that in non-COPD patients; nevertheless, ICU mortality increased by 17 % when COPD patients developed VAP, remaining an independent predictor of mortality. Length of ICU stay and duration of mechanical ventilation were also prolonged in COPD patients. Similar findings have been reported in the study of Makris et al. from a single ICU [26]. Compared to patients without COPD the prevalence of Pseudomonas aeruginosa VAP (overall and with early onset) was higher. Overall, the prevalence of non-fermenting Gram negative bacilli was higher in COPD patients (p = 0.056), especially in those with early-onset VAP.
Despite not being a risk factor for occurrence, older age proved to be an independent risk factor for mortality. The adverse effect of ageing has been previously reported in septic critically ill patients [33], but the particular effect of old age in VAP outcome has never been evaluated before, to the best of our knowledge [33].
PRMs were found in 50.7 % of patients with early-onset pneumonia and without known risk factors. Those patients received more frequent inappropriate empirical therapy and had a higher mortality. The presence of severe sepsis/septic shock and the hospitalization in ICUs with more than a 25 % prevalence of PRM were independently associated with the identification of PRM isolates. Given the importance of timely prescribing efficient antibiotics in order to reduce sepsis mortality [34], physicians working in ICUs with a high prevalence of PRM should empirically cover patients with severe sepsis and septic shock from nosocomial pneumonia against resistant pathogens, irrespective of the existence of known risk factors. Carbapenems, piperacillin/tazobactam, and fluoroquinolones, in declining order, were the most frequent antibiotics used in VAP. The main factors that influenced empirical prescription study were admission category, hospital ecology (prevalence of A. baumannii > 10 %) and length of stay more than 5 days. In agreement with previous studies [34], mortality was lower in patients who received appropriate initial treatment. To the best of our knowledge this is the first large-scale study describing antibiotic prescription for VAP in Europe, and since its publication no other study has shed further light on this issue.
The EU-VAP/CAP study did not find a relation between the staff levels, defined as the patient to nurse ratio, and the incidence of VAP. Higher nurse staffing levels in ICUs have been associated with better survival rates [35]. A hospital-wide systematic review concluded that the existing evidence neither confirms nor rules out an inverse relationship between nurse staffing and pneumonia rates [36]. Similarly with the EU-VAP/CAP study, a large multicenter study in German ICUs came to the same conclusion that nurse ratio did not affect VAP incidence [37]. A certain relationship probably exists, but the effect is rather discrete and easily confounded by other stronger risk factors, and thus further studies are needed for this effect to be elucidated.
The limitations of the EU-VAP/CAP study should be addressed. Participating centers were mainly from southern and central Europe, a fact that may render some of the results, especially about the microbiology of pneumonia, not applicable for Europe as a whole. Another limitation is that the majority of the participating ICUs were in university or university-affiliated hospitals, and this could pose a bias, as university departments might have a more intense use of invasive diagnostic procedures. Furthermore, diagnosis of pneumonia was clinical and some degree of objective selection might exist. Because data were collected in 2006, it would be interesting to assess evolution with a new cohort.
In summary, the EU-VAP/CAP study added new information on the European current management of nosocomial pneumonia in the ICU setting and answered questions about special subgroups and special situations. Our findings suggest that diagnosis of VAP should be supported by clinical signs rather than using CPIS score. Current management is different from guidelines, suggesting the need for a personalized approach. Our evidence can be used in improving future guidelines, and in the design of future randomized trials.
References
American Thoracic Society, Infectious Diseases Society of America (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416
Woodhead M, Welch CA, Harrison DA et al (2006) Community-acquired pneumonia on the intensive care unit: secondary analysis of 17,869 cases in the ICNARC Case Mix Programme Database. Crit Care 10:S1
Walden AP, Clarke GM, McKechnie S et al (2014) Patients with community acquired pneumonia admitted to European intensive care units: an epidemiological survey of the GenOSept cohort. Crit Care 18:R58
Koulenti D, Rello J (2006) Hospital-acquired pneumonia in the 21st century: a review of current treatment options and their impact on patient care. Expert Opin Pharmacother 7:1555–1569
Kalanuria AA, Zai W, Marek M (2014) Ventilator-associated pneumonia in the ICU. Crit Care 18:208
Rello J, Lisboa T, Koulenti D (2014) Respiratory infections in patients undergoing mechanical ventilation. Lancet Respir Med 2:764–744
Sopena N, Sabria M, Neunos 2000 Study Group (2000) Study group (2005) Multicentre study of hospital-acquired pneumonia in non-ICU patients. Chest 127:213–219
Ohi H, Yanagihara K, Miyazaki Y et al (2004) Hospital-acquired pneumonia in general wards of a Japanese tertiary hospital. Respirology 9:120–124
Cakir Edis E, Hatipoglu O, Yilmam I et al (2009) Hospital-acquired pneumonia developed in nonintensive care units. Respiration 78:416–422
Klompas M (2012) What can we learn from international-ventilator associated pneumonia rates? Crit Care Med 40:3303–3304
Koulenti D, Lisboa T, Brun-Buisson C et al (2009) Spectrum of practice in the diagnosis of nosocomial pneumonia in patients requiring mechanical ventilation in European intensive care units. Crit Care Med 37:2360–2368
Magret M, Amaya-Villar R, Garnacho J et al (2010) Ventilator-associated pneumonia in trauma patients is associated with lower mortality: results from EU-VAP study. J Trauma 69:849–854
Koulenti D, Blot S, Dulhunty JM et al (2015) COPD patients with ventilator-associated pneumonia: implications for management. Eur J Clin Microbiol Infect Dis 34:2403–2411
Blot S, Koulenti D, Dimopoulos G et al (2014) Prevalence, risk factors, and mortality for ventilator-associated pneumonia in middle-aged, old, and very old critically ill patients. Crit Care Med 42:601–609
Magret M, Lisboa T, Martin-Loeches I et al (2011) Bacteremia is an independent risk factor for mortality in nosocomial pneumonia: a prospective and observational multicenter study. Crit Care 15:R62
Martin-Loeches I, Deja M, Koulenti D et al (2013) Potentially resistant microorganisms in intubated patients with hospital-acquired pneumonia: the interaction of ecology, shock and risk factors. Intensive Care Med 39:672–681
Rello J, Ulldemolins M, Lisboa T et al (2011) Determinants of prescription and choice of empirical therapy for hospital-acquired and ventilator-associated pneumonia. Eur Respir J 37:1332–1339
Blot S, Serra ML, Koulenti D et al (2011) Patient to nurse ratio and risk of ventilator associated pneumonia in critically ill patients. Am J Crit Care 20:e1–e9
Rello J, Diaz E, Roque M, Vallés J (1999) Risk factors for developing pneumonia within 48 hours of intubation. Am J Respir Crit Care Med 159:1742–1746
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Vincent JL, Moreno R, Takala J et al (1996) The SOFA (Sepsis related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Cavalcanti M, Ferrer M, Ferrer R et al (2006) Risk and prognostic factors of ventilator-associated pneumonia in trauma patients. Crit Care Med 34:1067–1072
Magnotti LJ, Croce MA, Fabian TC (2004) Is ventilator-associated pneumonia in trauma patients an epiphenomenon or a cause of death? Surg Infect 5:237–242
Agbaht K, Lisboa T, Pobo A et al (2007) Management of ventilator-associated pneumonia in a multidisciplinary intensive care unit: does trauma make a difference? Intensive Care Med 33:1387–1395
Rodríguez A, Lisboa T, Solé-Violán J et al (2011) Impact of nonexacerbated COPD on mortality in critically ill patients. Chest 139:1354–1360
Makris D, Desrousseaux B, Zakynthinos E et al (2011) The impact of COPD on ICU mortality in patients with ventilator-associated pneumonia. Respir Med 105(7):1022–1029
Blot S, Cankurtaran M, Petrovic M et al (2009) Epidemiology and outcome of nosocomial bloodstream infection in elderly critically ill patients: a comparison between middle-aged, old, and very old patients. Crit Care Med 37:1634–1641
Bagshaw SM, Webb SA, Delaney A et al (2009) Very old patients admitted to intensive care in Australia and New Zealand: a multi-centre cohort analysis. Crit Care 13:R45
Boots RJ, Lipman J, Bellomo R et al (2005) The spectrum of practice in the diagnosis and management of pneumonia in patients requiring mechanical ventilation. Australian and New Zealand Practice in Intensive Care (ANZPIC II). Anaesth Intensive Care 33:87–100
Kollef MH, Morrow LE, Niederman MS et al (2006) Clinical characteristics and treatment patterns among patients with ventilator-associated pneumonia. Chest 129(5):1210–1218
Tabah A, Koulenti D, Laupland K et al (2012) Characteristics and determinants of outcome of hospital-acquired bloodstream infections in intensive care units: the EUROBACT International Cohort Study. Intensive Care Med 38(12):1930–1945
Timsit JF, Tabah A, Koulenti D et al (2014) Update of hospital-acquired bacteremia respiratory infection: experience from the EURO-BACT study. Clin Pulm Med 21:9–15
Martin GMD, Mannino DM, Moss M (2006) The effect of age on the development and outcome of adult sepsis. Crit Care Med 34:15–21
Kumar A, Roberts D, Wood KE et al (2006) Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 34:1589–1596
Cho SH, Hwang JH, Kim J (2008) Nurse staffing and patient mortality in intensive care units. Nurs Res 57(5):322–330
Lang TA, Hodge M, Olson V, Romano PS, Kravitz RL (2004) Nurse-patient ratios: a systematic review on the effects of nurse staffing on patient, nurse employee, and hospital outcomes. J Nurs Adm 34(7–8):326–337
Schwab F, Meyer E, Geffers C, Gastmeier P (2012) Understaffing, overcrowding, inappropriate nurse:ventilated patient ratio and nosocomial infections: which parameter is the best reflection of deficits? J Hosp Infect 80:133–139
Acknowledgments
EU-VAP/CAP Study was endorsed by the European Critical Care Research Network (ECCRN).
Conflict of interest
JR served in the speakers’ bureau or as consultant for Pfizer, Paratek, Astra-Zeneca, KENTA, Nabriva. DK and ET report no conflict of interest.
EU-VAP/CAP Study Investigators
Djilali Annane (Raymond Poincaré Univ. Hospital, Garches, France), Rosario Amaya-Villar (Virgen de Rocio Univ. Hospital, Seville, Spain), Apostolos Armaganidis (Attikon Univ. Hospital, Athens, Greece; National Coordinator [NC]), Stijn Blot (1,2Ghent Univ. Hospital, Ghent, Belgium, 1RBWH University of Queenland,), Christian Brun-Buisson (Henri-Mondor Univ. Hospital, Paris, France; NC), Silvano Cardellino (Cardinal Massaia Hospital, Asti, Italy), Antonio Carneiro (2Santo Antonio Hospital, Porto, Portugal; NC), Maria Deja (Charite Univ. Hospital, Berlin, Germany), Jan DeWaele (Ghent Univ. Hospital, Ghent, Belgium; NC), Emili Diaz (Joan XIII Univ. Hospital, CIBERES, Tarragona, Spain), George Dimopoulos (1,2Attikon Univ. Hospital, 2Sotiria Hospital, Athens, Greece), Jose Garnacho-Montero (Virgen de Rocio Univ. Hospital, Seville, Spain), Muhammet Guven (Erciyes Univ. Hospital, Kayseri, Turkey), Apostolos Komnos (Larisa Hospital, Larisa, Greece), Despoina Koulenti (Study Coordinator, 1,2Attikon Univ. Hospital, Athens, Greece, 1RBWH University of Queenland), Wolfgang Krueger (1Constance Hospital, Constance, Germany,2Tuebingen Univ. Hospital, Tuebingen, Germany; NC), Thiago Lisboa (2Joan XIII Univ. Hospital, Tarragona, Spain and CIBERES), Antonio Macor (Amedeo di Savoia Hospital, Torino, Italy; NC), Emilpaolo Manno (Maria Vittoria Hospital, Torino, Italy), Rafael Mañez (Bellvitge Univ. Hospital, Barcelona, Spain), Brian Marsh (Mater Misericordiae Univ. Hospital, Dublin, Ireland), Claude Martin (Nord Univ. Hospital, Marseille, France), Pavlos Myrianthefs (Aghioi Anargyroi Hospital1, KAT Hospital2, Athens, Greece), Marc Nauwynck (St Jan Hospital, Brugges, Belgium), Laurent Papazian (Hôpitaux de Marseille Aix-Marseille Université, 2Sainte-Marguerite Univ. Hospital, Marseille, France), Christian Putensen (Bonn Univ. Hospital, Bonn, Germany), Bernard Regnier (Claude Bernard Univ. Hospital, Paris, France), Jordi Rello (Principal Investigator, 1CIBERES, Universitat Autonoma de Barcelona, Spain, 2Joan XIII Univ. Hospital, Tarragona, Spain; NC), Jordi Sole-Violan (Dr Negrin Univ. Hospital, Gran Canarias, Spain), Giuseppe Spina (Mauriziano Umberto I Hospital, Torino, Italy), Arzu Topeli (Hacettepe Univ. Hospital, Ankara, Turkey; NC), Hermann Wrigge (Bonn Univ. Hospital, Bonn, Germany). [1Current affiliation, 2affiliation during the period of the study].
Funding
CIBERES (PCI Pneumonia) Instituto Salud Carlos III, Madrid.
Author information
Authors and Affiliations
Corresponding author
Additional information
The EU-VAP/CAP study of the WG on Pneumonia of the Infection Section of ESICM was a project endorsed by ESICM.
Rights and permissions
About this article
Cite this article
Koulenti, D., Tsigou, E. & Rello, J. Nosocomial pneumonia in 27 ICUs in Europe: perspectives from the EU-VAP/CAP study. Eur J Clin Microbiol Infect Dis 36, 1999–2006 (2017). https://doi.org/10.1007/s10096-016-2703-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2703-z