Abstract
The significant increase of the linezolid-resistant enterococci (LRE) has been observed in Polish hospitals since 2012 and our study aimed at elucidating the possible reasons for this phenomenon. Polish LRE isolates were analysed by multilocus-sequence typing (MLST) and multiple locus variable-number tandem repeat (VNTR) analysis (MLVA), polymerase chain reaction (PCR) and PCR-restriction fragment length polymorphism (PCR-RFLP) to establish clonal relatedness and mechanism of linezolid resistance, respectively. Fifty analysed LRE (2008–2015) included mostly Enterococcus faecium (82%) and Enterococcus faecalis (16%). Enterococcus faecium belonged to the hospital-adapted lineages 17/18 and 78, while E. faecalis isolates represented ST6, a hospital-associated type, and ST116, found in both humans and food-production animals. The G2576T 23S rRNA mutation was the most frequent (94%) mechanism of linezolid/tedizolid resistance of LRE. None of the isolates carried the plasmid-associated gene of Cfr methyltransferase, whereas optrA, encoding the ABC-type drug transporter, was identified in two E. faecalis isolates. In these isolates, optrA was located on a plasmid, transferable to both E. faecium and E. faecalis, whose partial (36.3 kb) sequence was 100% identical to the pE394 plasmid, identified previously in China in both clinical and farm animal isolates. The optrA–E. faecium transconjugant displayed a significant growth deficiency, in contrast to the optrA–E. faecalis. Our study indicates the role of mutation acquisition by hospital-adapted clones of enterococci as a major driver of increasing resistance to linezolid and tedizolid. Transferability and apparent lack of a biological cost of resistance suggest that E. faecalis may be a natural reservoir of optrA, an emerging mechanism of oxazolidinone resistance.
Similar content being viewed by others
Introduction
Although enterococci represent generally harmless commensals of humans and animals, they have recently become a common cause of hospital-associated infections (HAIs). The majority of enterococcal HAIs are caused by high-risk enterococcal clonal complexes (HiRECCs) of the two clinically relevant species, Enterococcus faecalis and Enterococcus faecium. HiRECCs of E. faecalis include clonal complexes (CCs) 6 (also known as CC2), 9 and 87 significantly over-represented among hospital strains [1, 2]. Almost all clinical isolates of E. faecium were initially included in CC17, subsequently divided into lineages 17, 18 and 78 [3]. The Bayesian analysis of population structure (BAPS) clustered nosocomial isolates into two groups, BAPS 2-1 and BAPS 3-3, corresponding to lineages 78 and 17/18, respectively [4].
The increasing prevalence of enterococci, resistant to important anti-enterococcal drugs, such as aminoglycosides and glycopeptides, currently represents a great challenge for clinicians. Linezolid was the first antimicrobial agent of the class of oxazolidinones introduced to clinical use to treat infections caused by multidrug-resistant aerobic Gram-positive bacteria, including vancomycin-resistant enterococci (VRE) [5]. Linezolid inhibits protein synthesis by binding the central loop of domain V in the 23S rRNA in bacterial ribosome [6] and in enterococci, the G2576T (Escherichia coli numbering) mutation in the 23S rRNA gene(s) was, thus far, the predominant mechanisms responsible for the loss of susceptibility to this compound. Other mechanisms of linezolid resistance include methylation of the A2503 residue in 23S rRNA by the Cfr methyltransferase and mutations in the L3 and L4 ribosomal proteins (reviewed recently in [7]). Recently, an efflux mechanism of resistance to oxazolidinones and phenicols was found in both E. faecalis and E. faecium [8], determined by the plasmid-borne optrA gene, responsible for the production of the ABC-type transporter OptrA.
Linezolid-resistant enterococci (LRE) as hospital alert pathogens are collected by the National Reference Centre for Susceptibility Testing (NRCST), located at the National Medicines Institute in Warsaw, Poland. Since 2012, LRE have been increasingly reported to the NRCST. Thus, we performed a study with the aim to characterise recent Polish clinical isolates of LRE with respect to their species composition, susceptibility phenotypes to other antimicrobials, mechanisms of linezolid resistance and clonal relatedness. The prevalence of optrA, its genetic environment, transferability and associated fitness cost were of our special interest due to their relevance for the spread of linezolid resistance in enterococci. To our knowledge, this is the first report of such data for LRE from Central-Eastern Europe and one of a very few showing the importance of the transferable optrA determinant.
Materials and methods
Species identification, antimicrobial susceptibility testing and analysis of vancomycin resistance determinants
Fifty clinical LRE isolates (one isolate per patient) were collected by the NRCST during the period from September 2008 till the end of December 2015 in passive surveillance. The isolates were obtained from 20 hospital settings, located in 12 Polish cities (Supplementary Fig. A1). Species identification was performed using standard microbiological methods and, for Enterococcus avium, by multilocus sequence analysis (MLSA) [9]. Total DNA was extracted using Genomic DNA Prep Plus (A&A Biotechnology, Gdynia, Poland). Isolates were stored at −80 °C until further analysis. Antimicrobial susceptibility of the isolates to daptomycin and tedizolid was evaluated with the use of stripes with predefined antibiotic concentrations (bioMérieux, Marcy-l’Etoile, France and Liofilchem, Roseto degli Abruzzi, Italy, respectively) and for the remaining antimicrobials (Table 1) by the broth microdilution method, according to the Clinical and Laboratory Standards Institute (CLSI) guidelines, using reference strains E. faecalis ATCC 29212 and Staphylococcus aureus ATCC 29213 as quality controls. Minimum inhibitory concentration (MIC) interpretation was conducted according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) or CLSI clinical breakpoints (when EUCAST breakpoints were not available) and the clinical breakpoint for tedizolid for E. faecalis was applied for all enterococcal isolates. The detection of vanA and vanB was performed by polymerase chain reaction (PCR) [10, 11], with E. faecium BM4147 and E. faecalis V583 as positive controls, respectively. The Tn1546 structure was investigated by PCR mapping [12,13,14,15,16,17].
Molecular typing of isolates
The clonal relatedness of E. faecium and E. faecalis was investigated using multilocus sequence typing (MLST) [1, 3] and the MLST database (http://pubmlst.org/; 1st February 2016, date last accessed). Sequence types (STs) were assigned to CCs with comparative eBURST analysis against the whole databases of E. faecium and E. faecalis (http://eburst.mlst.net/; 1st February 2016, date last accessed). E. faecium isolates were additionally investigated using multiple locus variable-number tandem repeat (VNTR) analysis (MLVA; http://www.umcutrecht.nl/en/Research/Miscellaneous/MLVA-typing; 1st August 2016, date last accessed).
Detection and analysis of linezolid resistance determinants
Isolates were examined for the presence of the G2576T mutation in the 23S rRNA genes by sequencing [18] and PCR-restriction fragment length polymorphism (PCR-RFLP) analysis with NheI [19] of individual copies of 23S rRNA genes of E. faecium and E. faecalis (primer sequences available upon request). The rplC and rplD genes encoding ribosomal proteins L3 and L4, respectively, were sequenced [20] and compared to the wild-type sequences from E. faecalis ATCC 29212 (CP008816.1), E. faecium DO (CP003583.1) and E. avium ATCC 14025 (ASWL01000001.1) strains. The cfr gene was searched for by PCR [21] using DNA of a cfr-positive clinical isolate of S. aureus from the laboratory collection as a control (unpublished data) and optrA and repB pE349 were detected by PCR with primers designed in the study (5′-TCAACCTTGAAAGGGGACAG/AGCCAAGAGCAGTTCTGACC-3′ and 5′-TGAATTAGCAGTCGCCAGTTTAG/TTACCGTTGGCAAATATTGTGTG-3′, respectively). The localisation of optrA was established with pulsed-field gel electrophoresis (PFGE)-S1/hybridisation analysis. To this end, total genomic DNA was purified in agarose plugs [10], subjected to S1 nuclease digestion (Takara Bio, Japan) and separated by PFGE [22], followed by blotting onto the Hybond-N+ membrane (GE, Healthcare, Buckinghamshire, UK) and hybridisation with the optrA probe, using the Amersham ECL Random-Prime Labelling and Detection System (GE Healthcare).
Conjugation experiments
Transferability of linezolid resistance was studied for the optrA-positive isolates using a highly efficient conjugation protocol [23] with E. faecalis OG1RF and E. faecium 64/3 as recipients.
Genomic sequencing and plasmidome of optrA–E. faecalis
Whole-genome sequencing (WGS) was performed with Ion Torrent PGM (Thermo Fisher Scientific, Waltham, MA, USA) or MiSeq (Illumina, San Diego, CA, USA) as an external service. Obtained reads were assembled using the CLC Genomic Workbench v.8 (CLC bio, Aarhus, Denmark) and joining of contigs was performed with PCR (primer sequences available upon request), followed by sequencing of the products. The presence of rep genes, unique and characteristic for families 1–19, was either analysed by PCR [24] or deduced from WGS data using the BLAST function in the CLC software.
Growth experiments
The growth ratios of optrA transconjugants and recipient strains as a measure of the bacterial fitness and plasmid cost [25] were determined by culturing tested strains in BHI broth with and without linezolid (8 mg/L) overnight, respectively, diluting to OD 0.01 in BHI broth without antibiotics and growing at 37 °C with shaking for 10 h. OD600 measurements were taken every 30 min. The test was done twice, in three biologically independent replicates. The number of colony-forming units (CFUs) in cultures of recipients and transconjugants from the beginning and end of the test (t0 and t10, respectively) was established by plating appropriate dilutions on BHI agar and the enumeration of colonies.
Nucleotide accession numbers
The nucleotide sequence of rplD of E. avium, the complete sequence of optrA-plasmid 1 from the KIEL E. faecalis isolate and a partial sequence of plasmid 2 from the same isolate were deposited in GenBank (accession numbers KX255697, KY513280 and KY513281, respectively).
Results
Bacterial isolates: epidemiological data, species, antimicrobial susceptibility and clonal relatedness
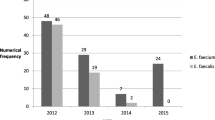
LRE were reported as single cases for 13 hospitals, while seven hospitals, located in Warsaw, Wroclaw, Poznan and Gdansk, sent up to 12 LRE each (Table 2, Supplementary Fig. A1). The most prevalent species, E. faecium (41 isolates, 82%), occurred in 16 hospitals, while E. faecalis (8 isolates, 16%) was obtained in four centres. One isolate was identified as E. avium. The first LRE was sent to the NRCST in September 2008, and, since 2012, the number of LRE per year has been generally increasing, in parallel to the number of affected hospitals (Table 2). Nearly half of the isolates were obtained from colonised patients (21; 42%); 15 and 14 isolates were from invasive and non-invasive infections, respectively. Linezolid MIC values ranged from 8 to 128 mg/L and all isolates were resistant to tedizolid (Table 1). All E. faecium and E. faecalis isolates were resistant to ciprofloxacin, susceptible to tigecycline and daptomycin and, in the case of E. faecium, to quinupristin–dalfopristin. Additionally, all E. faecium isolates displayed resistance to penicillin and ampicillin. In both species, a high prevalence of high-level aminoglycoside resistance (HLAR) was observed. Vancomycin resistance occurred only among E. faecium (37 isolates, 90.2%), mediated by the vanA and vanB gene clusters (34 and three isolates, respectively). All LRE exhibited the MDR phenotype (resistance to three or more classes of antimicrobials). Six E. faecalis isolates belonged to ST6, specific for HiRECC 6, and the remaining two isolates represented ST116 from CC116. Enterococcus faecium isolates represented eight related MLVA types (MTs) and 11 STs, characteristic for nosocomial lineages 17/18 and 78 (Table 2). Typing of Tn1546 of 20 vanA-positive E. faecium of MT12/ST117 from seven centres identified three transposon variants, A, B and C (Table 2).
Detection and analysis of linezolid resistance determinants
Sequencing of the 23S rRNA genes and the PCR-RFLP analysis of individual copies of 23S rDNA revealed the presence of the G2576T mutation for 47 isolates (94%); the remaining single isolate of E. faecium and two isolates of E. faecalis harboured the wild-type 23S rRNA genes (Table 2). The number of mutated copies of the 23S rRNA gene ranged from one to five out of six in E. faecium and from two to three out of four in E. faecalis. All isolates harboured the wild-type rplC gene. Mutation in the rplD gene was observed only for E. avium, where the T511G substitution resulted in the S171A change in the L4 deduced amino acid sequence. None of the isolates carried the cfr gene. Initially, optrA was detected in five isolates (10%), including two E. faecalis and three E. faecium. In the case of the three latter isolates, the gene was lost upon storage; thus, detailed analyses of optrA localisation and transferability could be continued only for E. faecalis. A remaining single isolate of E. faecium (linezolid MIC = 32 mg/L) did not contain any of the resistance determinants studied. The PFGE-S1 analysis of optrA-positive E. faecalis isolates showed the presence of two or three plasmids, ranging from <50 to ∼100 kb in size, and the subsequent Southern blot analysis showed the localisation of optrA on a plasmid below 50 kb in both cases. optrA was transferable by conjugation to both E. faecalis and E. faecium recipients. Enterococcus faecium transconjugants harboured a single plasmid of the size corresponding to the optrA plasmid from clinical isolates, while in E. faecalis transconjugants, either the optrA plasmid was accompanied by another plasmid, also below 50 kb in size, or a single ∼100-kb plasmid was observed. WGS of an optrA–E. faecalis transconjugant (donor: isolate from 2013 from a BYD hospital) with a ∼100-kb presumably recombinant plasmid yielded five contigs (sequencing coverage 86–140×), absent from the OG1RF genomic sequences (accession no. NC_017316). Two of these contigs were successfully joined by PCR into a 36,331-bp contig, 100% identical to the corresponding part of the conjugative plasmid pE349 carrying optrA (accession no. KP399637) [8] and characterised by the presence of the unique repB pE349 gene. The remaining three contigs showed a partial homology to the region encompassing 1.2–41.6 kb of the pTEF2 plasmid (accession no. AE016831; data not shown). In the donor isolate, PCR analysis revealed the presence of rep9 and rep17 genes. In growth experiments, an optrA–E. faecium transconjugant was characterised by a significantly delayed entrance into the logarithmic phase of growth, compared to the E. faecium 64/3 recipient, as well as to an optrA–E. faecalis transconjugant and the E. faecalis OG1RF recipient (Fig. 1). WGS of the other optrA clinical isolate (obtained in 2012 in a KIEL hospital) demonstrated that two contigs (35,000 bp and 1526 bp, coverage 178× and 329×, respectively) had sequences identical to pE349. These two contigs were successfully joined by PCR and sequencing of the products into a single circular plasmid, 36,331 bp in size (Supplementary Fig. 2A). This plasmid was also transferable by conjugation to both E. faecalis and E. faecium. The BLAST analyses of genomic sequences of the KIEL E. faecalis isolate revealed solely the presence of a representative of rep9 family, with the highest homology (94.0–96.6%) to prgW from pBEE99 (GU046453; [26]) and to repA genes of pMG2200 (AB374546; [27]) and pTEF2 (AE016831; [28]). This gene was located in a 20,525-bp contig (coverage 105×). This contig, presumably representing a part of a plasmid, showed similarity to pheromone-responsive plasmids, such as pBEE99 and to some Gram(+) integrative-conjugative elements, such as Tn6248 (KP834592), found in a pig isolate of E. faecium in China (Supplementary Fig. 2B).
Discussion
Linezolid is an important agent in the treatment of serious infections caused by multidrug-resistant Gram-positive organisms, including methicillin-resistant S. aureus (MRSA) and VRE. The first case of LRE in Poland occurred in July 2003, shortly after the introduction of linezolid for treatment in our country in February 2002 [29]. Although LRE remain relatively rare in Poland [30], their increasing number is reported to the NRCST, along with a growing number of affected hospitals. While in systematic surveillance a stable low prevalence of linezolid resistance in Gram-positive bacteria, including enterococci, is observed [7], some other studies show a local increase of LRE, e.g. in Germany [31], similar to the situation observed in Poland. In our study, over 70% of LRE were isolated from patients of intensive care units (ICUs) and haematology wards, which are wards with the highest use of antimicrobials, including linezolid. LRE occurred mostly as sporadic cases, in agreement with the previous observations about the nature of LRE emergence [32]. Nevertheless, in some centres, the epidemic spread of LRE was observed, as indicated by a similar time of isolation, the same genotype and mechanism of resistance. Linezolid resistance was identified almost exclusively in two enterococcal species, which are the most frequent causes of enterococcal HAIs, E. faecalis and E. faecium. The linezolid-resistant E. faecium showed much higher prevalence of vancomycin resistance (90.2%), than typically found in HAIs in Polish hospitals for this species (7.1%) [30]. The analysis of clonality, resistance determinants and the Tn1546 structure indicated possible VRE/LRE outbreaks and even presumable transfer(s) among hospitals. The combination of these two resistance phenotypes in the background of multiresistant epidemic hospital meroclone is especially worrisome due to the lack of effective treatment options, and highlights the importance of active surveillance and infection control procedures.
The G2576T mutation within the domain V of 23S rRNA was the most frequent mechanism of linezolid resistance developed by Polish LRE, as frequently reported for enterococcal clinical isolates [7]. None of the isolates, however, harboured the G2576T mutation in all copies of 23S rDNA, which indicates that lack of the wild-type copy might impose too big a fitness cost for the cell [33]. The second mutational mechanism of resistance, was the T511G mutation in rplD (the S171A substitution in the L4 ribosomal protein) identified in our study for E. avium. The S/P mutation at the same position in a clinical E. avium isolate also from Poland constituted the only mechanism responsible for its elevated MIC of linezolid [7]. The only non-mutational mechanism of linezolid resistance detected in our study was the presence of ABC transporter OptrA [8]. Another linezolid resistance determinant, cfr, was not found, although its presence was observed for both E. faecalis and E. faecium [20, 34]. The optrA gene was first identified on the pE349 plasmid [8] and detected in human and animal E. faecalis and E. faecium from China, Malaysia, Austria, Ireland and Italy [8,36,, 35–37]. In our study, the optrA gene was the only determinant of linezolid resistance detected in two epidemiologically unrelated non-HiRECC E. faecalis of ST116. We also observed a loss of this determinant from three isolates of E. faecium, which additionally carried the G2576T mutation. A loss of conjugative resistance plasmids was reported by others [25]. Such instability might explain the generally much lower prevalence of optrA in E. faecium compared to E. faecalis [8, 38], and would be in agreement with the fitness cost imposed by an optrA plasmid on E. faecium transconjugant, observed in this study. The optrA-carrying plasmid was transferable from E. faecalis to both E. faecium and E. faecalis recipients, alone or with a second plasmid, respectively. The optrA plasmids of E. faecalis characterised so far are typically conjugative [8]. It is tempting to speculate about a possible source of the optrA gene, located on pE349, for clinical enterococci in Poland. As previously suggested, E. faecalis ST116, characteristic for isolates from both human infections as well as food-producing animals and retail meat [8, 39], may act as a vehicle of antimicrobial resistance between environment and hospital, similarly to vancomycin-resistant E. faecalis from imported turkey meat and clinical isolate, which also shared this ST [39]. Moreover, three isolates of E. faecalis from poultry, carrying pE349-like plasmids with the optrA gene, were reported recently from Columbia [40], indicating a global spread of optrA plasmids and supporting the potential role of an animal reservoir. Thus, a food-borne E. faecalis with pE349 could serve as a potential source of optrA for nosocomial enterococci, raising a new aspect for food safety.
In conclusion, we performed an extensive analysis of linezolid- and tedizolid-resistant isolates of Enterococcus spp., obtained recently from carriage and HAIs in Polish hospitals. The emergence of LRE was predominantly caused by independent de novo resistance development in various enterococcal strains, mostly representing HiRECCs, for which acquisition of linezolid resistance may be a next step in their evolution as hospital ‘superbugs’. While the mutation within the 23S rRNA gene was the most common determinant of resistance, the detection of the multiresistance gene optrA among Polish LRE and evidence for its in vitro transferability raises further concerns about the possible decreasing effectiveness of linezolid treatment in the future.
References
Ruiz-Garbajosa P, Bonten MJ, Robinson DA, Top J, Nallapareddy SR, Torres C, Coque TM, Cantón R, Baquero F, Murray BE, del Campo R, Willems RJ (2006) Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. J Clin Microbiol 44:2220–2228
Kawalec M, Pietras Z, Daniłowicz E, Jakubczak A, Gniadkowski M, Hryniewicz W, Willems RJ (2007) Clonal structure of Enterococcus faecalis isolated from Polish hospitals: characterization of epidemic clones. J Clin Microbiol 45:147–153
Willems RJ, van Schaik W (2009) Transition of Enterococcus faecium from commensal organism to nosocomial pathogen. Future Microbiol 4:1125–1135. doi:10.2217/fmb.09.82
Willems RJ, Top J, van Schaik W, Leavis H, Bonten M, Sirén J, Hanage WP, Corander J (2012) Restricted gene flow among hospital subpopulations of Enterococcus faecium. MBio 3:e00151-12. doi:10.1128/mBio.00151-12
Bozdogan B, Appelbaum PC (2004) Oxazolidinones: activity, mode of action, and mechanism of resistance. Int J Antimicrob Agents 23:113–119
Kloss P, Xiong L, Shinabarger DL, Mankin AS (1999) Resistance mutations in 23 S rRNA identify the site of action of the protein synthesis inhibitor linezolid in the ribosomal peptidyl transferase center. J Mol Biol 294:93–101
Mendes RE, Deshpande LM, Jones RN (2014) Linezolid update: stable in vitro activity following more than a decade of clinical use and summary of associated resistance mechanisms. Drug Resist Updat 17:1–12. doi:10.1016/j.drup.2014.04.002
Wang Y, Lv Y, Cai J, Schwarz S, Cui L, Hu Z, Zhang R, Li J, Zhao Q, He T, Wang D, Wang Z, Shen Y, Li Y, Feßler AT, Wu C, Yu H, Deng X, Xia X, Shen J (2015) A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in Enterococcus faecalis and Enterococcus faecium of human and animal origin. J Antimicrob Chemother 70:2182–2190. doi:10.1093/jac/dkv116
Naser SM, Thompson FL, Hoste B, Gevers D, Dawyndt P, Vancanneyt M, Swings J (2005) Application of multilocus sequence analysis (MLSA) for rapid identification of Enterococcus species based on rpoA and pheS genes. Microbiology 151:2141–2150
Clark NC, Cooksey RC, Hill BC, Swenson JM, Tenover FC (1993) Characterization of glycopeptide-resistant enterococci from U.S. hospitals. Antimicrob Agents Chemother 37:2311–2317
Dahl KH, Simonsen GS, Olsvik O, Sundsfjord A (1999) Heterogeneity in the vanB gene cluster of genomically diverse clinical strains of vancomycin-resistant enterococci. Antimicrob Agents Chemother 43:1105–1110
Palepou MF, Adebiyi AM, Tremlett CH, Jensen LB, Woodford N (1998) Molecular analysis of diverse elements mediating VanA glycopeptide resistance in enterococci. J Antimicrob Chemother 42:605–612
Huh JY, Lee WG, Lee K, Shin WS, Yoo JH (2004) Distribution of insertion sequences associated with Tn1546-like elements among Enterococcus faecium isolates from patients in Korea. J Clin Microbiol 42:1897–1902
Tsai JC, Hsueh PR, Chen HJ, Tseng SP, Chen PY, Teng LJ (2005) The erm(T) gene is flanked by IS1216V in inducible erythromycin-resistant Streptococcus gallolyticus subsp. pasteurianus. Antimicrob Agents Chemother 49:4347–4350
Talebi M, Pourshafie MR, Katouli M, Möllby R (2008) Molecular structure and transferability of Tn1546-like elements in Enterococcus faecium isolates from clinical, sewage, and surface water samples in Iran. Appl Environ Microbiol 74:1350–1356. doi:10.1128/AEM.02254-07
Jensen LB, Ahrens P, Dons L, Jones RN, Hammerum AM, Aarestrup FM (1998) Molecular analysis of Tn1546 in Enterococcus faecium isolated from animals and humans. J Clin Microbiol 36:437–442
Yu HS, Seol SY, Cho DT (2003) Diversity of Tn1546-like elements in vancomycin-resistant enterococci isolated from humans and poultry in Korea. J Clin Microbiol 41:2641–2643
Tsiodras S, Gold HS, Sakoulas G, Eliopoulos GM, Wennersten C, Venkataraman L, Moellering RC, Ferraro MJ (2001) Linezolid resistance in a clinical isolate of Staphylococcus aureus. Lancet 358:207–208
Woodford N, Tysall L, Auckland C, Stockdale MW, Lawson AJ, Walker RA, Livermore DM (2002) Detection of oxazolidinone-resistant Enterococcus faecalis and Enterococcus faecium strains by real-time PCR and PCR-restriction fragment length polymorphism analysis. J Clin Microbiol 40:4298–4300
Diaz L, Kiratisin P, Mendes RE, Panesso D, Singh KV, Arias CA (2012) Transferable plasmid-mediated resistance to linezolid due to cfr in a human clinical isolate of Enterococcus faecalis. Antimicrob Agents Chemother 56:3917–3922. doi:10.1128/AAC.00419-12
Kehrenberg C, Schwarz S, Jacobsen L, Hansen LH, Vester B (2005) A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503. Mol Microbiol 57:1064–1073
Barton BM, Harding GP, Zuccarelli AJ (1995) A general method for detecting and sizing large plasmids. Anal Biochem 226:235–240
Manson JM, Hancock LE, Gilmore MS (2010) Mechanism of chromosomal transfer of Enterococcus faecalis pathogenicity island, capsule, antimicrobial resistance, and other traits. Proc Natl Acad Sci U S A 107:12269–12274. doi:10.1073/pnas.1000139107
Jensen LB, Garcia-Migura L, Valenzuela AJ, Løhr M, Hasman H, Aarestrup FM (2010) A classification system for plasmids from enterococci and other Gram-positive bacteria. J Microbiol Methods 80:25–43. doi:10.1016/j.mimet.2009.10.012
Porse A, Schønning K, Munck C, Sommer MO (2016) Survival and evolution of a large multidrug resistance plasmid in new clinical bacterial hosts. Mol Biol Evol 33:2860–2873
Coburn PS, Baghdayan AS, Craig N, Burroughs A, Tendolkar P, Miller K, Najar FZ, Roe BA, Shankar N (2010) A novel conjugative plasmid from Enterococcus faecalis E99 enhances resistance to ultraviolet radiation. Plasmid 64:18–25. doi:10.1016/j.plasmid.2010.03.001
Zheng B, Tomita H, Inoue T, Ike Y (2009) Isolation of VanB-type Enterococcus faecalis strains from nosocomial infections: first report of the isolation and identification of the pheromone-responsive plasmids pMG2200, encoding VanB-type vancomycin resistance and a Bac41-type bacteriocin, and pMG2201, encoding erythromycin resistance and cytolysin (Hly/Bac). Antimicrob Agents Chemother 53:735–747. doi:10.1128/AAC.00754-08
Paulsen IT, Banerjei L, Myers GS, Nelson KE, Seshadri R, Read TD, Fouts DE, Eisen JA, Gill SR, Heidelberg JF, Tettelin H, Dodson RJ, Umayam L, Brinkac L, Beanan M, Daugherty S, DeBoy RT, Durkin S, Kolonay J, Madupu R, Nelson W, Vamathevan J, Tran B, Upton J, Hansen T, Shetty J, Khouri H, Utterback T, Radune D, Ketchum KA, Dougherty BA, Fraser CM (2003) Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science 299:2071–2074
Krawczyk B, Samet A, Bronk M, Hellmann A, Kur J (2004) Emerging linezolid-resistant, vancomycin resistant Enterococcus faecium from a patient of a haematological unit in Poland. Pol J Microbiol 53:193–196
Gawryszewska I, Żabicka D, Bojarska K, Malinowska K, Hryniewicz W, Sadowy E (2016) Invasive enterococcal infections in Poland: the current epidemiological situation. Eur J Clin Microbiol Infect Dis 35:847–856. doi:10.1007/s10096-016-2607-y
Klare I, Fleige C, Geringer U, Thürmer A, Bender J, Mutters NT, Mischnik A, Werner G (2015) Increased frequency of linezolid resistance among clinical Enterococcus faecium isolates from German hospital patients. J Glob Antimicrob Resist 3:128–131. doi:10.1016/j.jgar.2015.02.007
Kainer MA, Devasia RA, Jones TF, Simmons BP, Melton K, Chow S, Broyles J, Moore KL, Craig AS, Schaffner W (2007) Response to emerging infection leading to outbreak of linezolid-resistant enterococci. Emerg Infect Dis 13:1024–1030. doi:10.3201/eid1307.070019
Meka VG, Gold HS, Cooke A, Venkataraman L, Eliopoulos GM, Moellering RC Jr, Jenkins SG (2004) Reversion to susceptibility in a linezolid-resistant clinical isolate of Staphylococcus aureus. J Antimicrob Chemother 54:818–820
Patel SN, Memari N, Shahinas D, Toye B, Jamieson FB, Farrell DJ (2013) Linezolid resistance in Enterococcus faecium isolated in Ontario, Canada. Diagn Microbiol Infect Dis 77:350–353. doi:10.1016/j.diagmicrobio.2013.08.012
Brenciani A, Morroni G, Vincenzi C, Manso E, Mingoia M, Giovanetti E, Varaldo PE (2016) Detection in Italy of two clinical Enterococcus faecium isolates carrying both the oxazolidinone and phenicol resistance gene optrA and a silent multiresistance gene cfr. J Antimicrob Chemother 71:1118–1119. doi:10.1093/jac/dkv438
Valentin T, Leitner E, Valentin A, Krause R, Hopkins KL, Meunier D, Woodford N, Zollner-Schwetz I (2016) Clinical Enterococcus faecalis isolate carrying the novel oxazolidinone resistance gene optrA identified in Austria. In: Proceedings of the 26th European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), Amsterdam, Netherlands, April 2016. The European Society of Clinical of Microbiology and Infectious Disease, Basel, poster PLB46B
Mendes RE, Hogan PA, Jones RN, Sader HS, Flamm RK (2016) Surveillance for linezolid resistance via the Zyvox® Annual Appraisal of Potency and Spectrum (ZAAPS) programme (2014): evolving resistance mechanisms with stable susceptibility rates. J Antimicrob Chemother 71:1860–1865. doi:10.1093/jac/dkw052
Cai J, Wang Y, Schwarz S, Lv H, Li Y, Liao K, Yu S, Zhao K, Gu D, Wang X, Zhang R, Shen J (2015) Enterococcal isolates carrying the novel oxazolidinone resistance gene optrA from hospitals in Zhejiang, Guangdong, and Henan, China, 2010–2014. Clin Microbiol Infect 21:1095.e1–1095.e4. doi:10.1016/j.cmi.2015.08.007
Agersø Y, Lester CH, Porsbo LJ, Orsted I, Emborg HD, Olsen KE, Jensen LB, Heuer OE, Frimodt-Møller N, Aarestrup FM, Hammerum AM (2008) Vancomycin-resistant Enterococcus faecalis isolates from a Danish patient and two healthy human volunteers are possibly related to isolates from imported turkey meat. J Antimicrob Chemother 62:844–845. doi:10.1093/jac/dkn271
Cavaco LM, Bernal JF, Zankari E, Léon M, Hendriksen RS, Perez-Gutierrez E, Aarestrup FM, Donado-Godoy P (2016) Detection of linezolid resistance due to the optrA gene in Enterococcus faecalis from poultry meat from the American continent (Colombia). J Antimicrob Chemother. doi:10.1093/jac/dkw490
Acknowledgements
We thank all microbiologists who provided isolates to the National Reference Centre for Susceptibility Testing in Warsaw, Joanna Empel for a cfr+ isolate of Staphylococcus aureus, Janusz Fiett for helpful discussions and Zdzisław Markiewicz for the critical reading of the manuscript. This publication made use of the Enterococcus faecium MLST website (http://pubmlst.org/efaecium/) and of the Enterococcus faecalis MLST website (http://pubmlst.org/efaecalis/), sited at the University of Oxford and funded by the Wellcome Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Isolates were collected as a part of routine hospital surveillance. Ethical approval and informed consent were, thus, not required.
Funding
This study was supported in part by the grant Narodowy Program Ochrony Antybiotyków (NPOA) from the Polish Ministry of Health and by the grant SPUB MIKROBANK from the Polish Ministry of Science and Higher Education.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below are the links to the electronic supplementary material.
ESM 1
Distribution of LRE in Poland, 2008–2015. The sizes of the circles are proportional to the number of LRE identified in each city; the number of LRE identified in particular hospitals in a city is indicated within the circles; cities from which optrA-positive isolates were submitted are underlined. (PDF 41 kb)
ESM 2
Plasmids from the KIEL E. faecalis isolate. A. The structure of the optrA plasmid, 100% homologous to pE347 [KP399637]. B. The partial structure of the rep9 plasmid, with the regions with homology to pBEE99 [GU046453] and Tn6248 [KP834592] marked by solid and dashed arrows, respectively. Plasmid replication genes in blue, conjugation genes in green, antimicrobial resistance genes in red, other genes/genes of unknown function in yellow. The UGENE software (Okonechnikov K, Golosova O, Fursov M, the UGENE team. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics 2012 28:1166–1167. doi:10.1093/bioinformatics/bts091) was used to visualise ORFs, followed by manual editing of the figure. (PDF 176 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gawryszewska, I., Żabicka, D., Hryniewicz, W. et al. Linezolid-resistant enterococci in Polish hospitals: species, clonality and determinants of linezolid resistance. Eur J Clin Microbiol Infect Dis 36, 1279–1286 (2017). https://doi.org/10.1007/s10096-017-2934-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-017-2934-7