Abstract
Positive serology for dengue and/or scrub typhus infection with/without positive malarial smear (designated as mixed or co-infection) is being increasingly observed during epidemics of acute undifferentiated febrile illnesses (AUFIs). We planned to study the clinical and biochemical spectrum of co-infections with Plasmodium sp., dengue virus and scrub typhus and compare these with mono-infection by the same organisms. During the period from December 2012 to December 2013, all cases presenting with AUFIs to a single medical unit of a referral centre in Garhwal region of the north Indian state of Uttarakhand were retrospectively selected and categorised aetiologically as co-infections, malaria, dengue or scrub typhus. The groups thus created were compared in terms of demographic, clinical, biochemical and outcome parameters. The co-infection group (n = 49) was associated with milder clinical manifestations, fewer, milder and non-progressive organ dysfunction, and lesser need for intensive care, mechanical ventilation and dialysis as compared to mono-infections. When co-infections were sub-grouped and compared with the relevant mono-infections, there were differences in certain haematological and biochemical parameters; however, this difference did not translate into differential outcomes. Scrub typhus mono-infection was associated with severe disease in terms of both morbidity and mortality. Malaria, dengue and scrub typhus should be routinely tested in all patients with AUFIs. Co-infections, whether true or due to serological cross-reactivity, appear to be a separate entity so far as presentation and morbidity is concerned. Further insight is needed into the mechanism and identification of the protective infection.
Similar content being viewed by others
Introduction
Co-infections with harmful [1, 2] and beneficial [3, 4] interactions came to the fore in the human immunodeficiency virus (HIV) era. HIV–malaria dual infections are well recognised in sub-Saharan Africa [5]. Co-infections with Gram-negative bacteria in malaria-infected individuals produce detrimental effects [6], while Ascaris lumbricoides co-infection is apparently protective from cerebral malaria [7]. Co-infections are being increasingly recognised in recent times in immune-competent patients. Dual infections with leptospira and scrub typhus have been described in Thai agricultural workers exposed to both infections by virtue of their occupation [8]. Dual infections are being increasingly reported from the northern states of India witnessing unprecedented epidemics of acute febrile illnesses [9].
Tropical diseases, namely malaria, dengue, scrub typhus and enteric fever, are seen pan-India throughout the year. Epidemics of acute undifferentiated febrile illnesses (AUFIs) due to malaria, dengue, scrub typhus, enteric fever and leptospirosis occur during and immediately after the monsoons with considerable regularity. The patients usually present with acute-onset fever with or without headache, cough, abdominal pain, conjunctival injection, lymphadenopathy or rash in most instances. Due to the overlapping clinical and laboratory features, it is prudent to test the entire differential diagnosis. During the 2012–2013 epidemic of AUFI, we detected a few cases with positive serology for dengue and/or scrub typhus and/or positive smear for malaria (designated here as co-infections).
We hypothesised that the patients with co-infections would have greater morbidity and higher mortality than those with mono-infections because of the summative effects of the individual infections on the various organ systems of our body. The aim of this study was to test this hypothesis by comparing the clinical details and outcome of patients admitted with mono- and co-infections. We also wished to study the magnitude and pattern of co-infections as a cause of AUFIs.
Materials and methods
Study setting
Uttarakhand is predominantly a mountainous state in northern India and 65 % of its area is covered with forests. It has Tibet to the north, Nepal to the east and the Indian states of Uttar Pradesh and Himachal Pradesh to the south and north-west, respectively. The Ganges is the major river system and the varied vegetation includes alpine meadows, sub-alpine conifer and sub-tropical pine forests, moist deciduous forests and grasslands. It has a population of over 10 million, of which 70 % live in rural areas. Uttarakhand has two major divisions: Garhwal comprising seven north-western districts and the Kumaon division comprising six districts. The Himalayan Hospital is a 900-bed tertiary care teaching hospital affiliated to the Himalayan Institute of Medical Sciences located 25 km from Dehradun, the capital of Uttarakhand. The hospital mainly caters the Garhwal division, some districts of the Kumaon division and the densely populated adjoining districts of Uttar Pradesh.
Patient selection
The present study was a retrospective observational study on all patients with AUFI (<2 weeks duration of fever and no localising features of infection) presenting to a single unit of the Department of Medicine between December 2012 and December 2013. Patients admitted to a single unit were included to maintain uniformity in decisions of hospitalisation, investigations, management and discharge. They were included if they fulfilled the diagnostic criteria of one or more than one infectious processes as outlined below; those without a clinical diagnosis were categorised as unknown infections and excluded.
In the presence of consistent clinical features, acute malaria was diagnosed by Leishman stained smear positivity, dengue by positive IgM serology (rapid card test, SD Bioline; sensitivity 94.2 %, specificity 96.4 %) and scrub typhus by positive IgM enzyme-linked immunosorbent assay (ELISA) (InBios International, Inc., USA; sensitivity 99 %, specificity 97.5 %). Besides these tests, a few cases were subjected to hepatitis A, B and E viral serologies, where evidence of acute hepatitis was present. All tests were performed in a National Accreditation Board of Laboratories (NABL)-accredited lab with stringent protocol-based quality control. Those without an aetiological diagnosis were excluded from the study.
The patients thus selected were categorised as co-infection (if they demonstrated positivity of more than one infectious aetiology as outlined above), else as mono-infection with acute malaria, dengue fever or scrub typhus according to the investigation results. The co-infection was further sub-categorised as M + D (malaria and dengue co-infection), D + S (dengue and scrub typhus co-infection), M + S (malaria and scrub co-infection) and M + D + S (malaria, dengue and scrub typhus co-infection).
Data collection
Clinical information including the duration of fever, associated symptoms (nausea, vomiting, headache, loose stools, breathlessness, abdominal pain, bleeding manifestations and the site of bleed, if present, seizures, unconsciousness, oliguria and swelling over the body etc.) and signs (heart rate, blood pressure, respiratory rate, oxygen saturation, palpable organomegaly and other abnormal clinical findings) was collected at the time of presentation in the hospital for all patients included in the study. Demographic details including age, gender and occupation were also collected and compiled. The haematological and biochemical investigations carried out at the time of hospitalisation were also noted. Outcomes studied were mortality and morbidity (the duration of hospitalisation, number of organs affected, time to recovery of platelets and the need for intensive care, mechanical ventilation and dialysis).
Renal impairment (renal failure) was defined as oliguria with acidosis and/or a rise in blood urea nitrogen (BUN) and serum creatinine as per our laboratory normal values. An increase in BUN or oliguria, in isolation, was not considered as a marker of renal failure. Respiratory involvement (respiratory distress) was defined as tachypnoea (>20/min) along with a fall in oxygen saturation to <90 %. Liver dysfunction was defined as a two-fold rise in alanine transaminases (normal value: 10–40 IU/l); isolated hyperbilirubinaemia, i.e. 1.5–6 mg/dl, was not attributed to liver dysfunction if liver transaminases were within normal limits. Central nervous system involvement was considered if single or multiple episodes of generalised tonic–clonic seizures were reported or the subject was in altered sensorium and/or disoriented at presentation. Increase in platelet count in consecutive samples, or beyond 50,000/mm3 when less at presentation, was considered to be recovery of platelets [10].
Data analysis
Data were analysed using the statistical software SPSS version 22. Qualitative data were presented in the form of frequency and percentage; quantitative data were presented as mean ± standard deviation (SD). It was observed that the data were not normally distributed, so median and inter-quartile values (IQR) were calculated. The Kruskal–Wallis H test was used to compare the difference among groups, followed by the Mann–Whitney U test to compute the differences between the groups. A p-value < 0.05 was considered as being statistically significant. Ethical approval was not required as it was an observational study.
Results
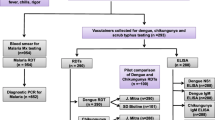
Of the 298 cases hospitalised for AUFIs, 233 patients were included in the study, and the rest were excluded as the infections remained undiagnosed. In the mono-infection groups, acute malaria (n = 51) was caused by Plasmodium vivax in 63.9 % (n = 39) of cases, P. falciparum in 27.8 % (n = 17) and by both the species in the rest. In addition to positive IgM antibodies (n = 58) for dengue fever, the NS1 antigen and IgG was positive in 44 (75.8 %) and 6 (10.3 %) cases, respectively. IgM antibodies to Orientia sp. were detected in all cases (n = 65) with scrub typhus mono-infection.
Forty-nine patients (16.4 %) had evidence of infection by more than one organism. Scrub typhus (n = 40), dengue (n = 35) and malaria (n = 28) were seen in various combinations and considered for the study (see Fig. 1). Hepatitis A virus co-infection was observed in two cases with positive scrub and dengue serologies. Hepatitis E co-infection was seen in a case co-infected with P. vivax and P. falciparum, and scrub typhus. Salmonella typhi was also cultured from a case infected with other pathogens, while the Widal test was strongly positive in two more cases. Plasmodium falciparum (n = 14, 50 %) was the dominant Plasmodium sp. in the group with co-infection, while P. vivax was observed in 9 cases (32.1 %); both the species co-infected in 5 (17.8 %) cases.
Scrub typhus mono-infection had a significantly longer mean duration of fever and higher white cell and platelet counts and alkaline phosphatase levels as compared to all of the three major aetiological groups. A significant difference from malaria was seen in terms of liver transaminases and serum bilirubin levels, while haemoglobin, bilirubin and creatinine were significantly higher than dengue mono-infection (p < 0.05). The co-infection group as a whole differed from scrub typhus in creatinine and transaminase levels additionally. Differences with malaria were also observed in platelet counts, liver transaminases and bilirubin. Also, a longer mean duration of fever, higher creatinine and bilirubin but lower liver enzymes were observed when compared with dengue mono-infection (p < 0.05).
As the co-infection group was heterogeneously composed of varying combinations of mono-infections by malaria, scrub and dengue, we compared the various co-infection sub-groups with the relevant mono-infections in terms of biochemical, haematological and outcome measures wherever possible. Utilising the Kruskal–Wallis test, we observed that all seven sub-groups (M + D, M + S, D + S, M + D + S and the three mono-infections) differed significantly in relation to the duration of fever and hospitalisation, haemoglobin, total leucocyte count (TLC), platelet counts, liver enzymes and serum creatinine; however, no difference was observed in the platelet recovery time, duration of hospitalisation and organ dysfunction. However, upon comparing the co-infections with their relevant mono-infections, there were differences in certain haematological and biochemical parameters (see Table 1).
The prominent clinical features of the aetiological groups overlapped considerably. Bleeding manifestations were mainly in the form of melena and petechial haemorrhages; haematemesis, haemoptysis and haematuria occurred in just one instance each. Though nearly one-fifth and a third of those with co-infections and scrub typhus reported breathlessness at presentation, less than half of these required ventilator and/or intensive care (see Table 2). Peripheral circulatory failure was observed in four patients with scrub typhus and two patients each with malaria and dengue, respectively; capillary leak was seen in dengue mono-infection (n = 31; 53.4 %) but was conspicuously absent in co-infections of dengue.
Intensive care was required in 4, 3, 3 and 16 cases with co-infections, malaria, dengue and scrub typhus, respectively. The duration of hospitalisation was longest and the organ dysfunction was significantly more as compared to the mono-infections and co-infections as a whole (p < 0.05). Platelets were transfused predominantly in patients with malaria and co-infections, though the bleeding manifestations were comparable in all of the aetiological groups. Mortality was low in all major aetiological groups except scrub typhus, where nearly 10 % succumbed. Abortions and intra-uterine deaths occurred in 2/7, 1/3, 0/1 and 2/4 pregnant females with co-infections, malaria, dengue and scrub typhus in their second and last trimesters, respectively. However, there was no statistical difference in terms of morbidity when co-infections were analysed with the relevant mono-infections (see Table 3).
Discussion
AUFI with evidence of co-infection by more than one organism (namely Plasmodium sp., dengue virus and Orientia tsutsugamushi) is a prominent group responsible for nearly one-sixth of all cases of AUFIs in our study. The features and outcomes of this heterogeneous group when considered as a whole were different from mono-infection by the same aetiological agents. The presentation and severity appeared to be mild when compared to mono-infections and there was no summation of effects of the different infectious agents as hypothesised. However, the differences in certain biochemical parameters when the specific co-infections were compared with the relevant co-infections did not translate into significantly different outcomes in terms of mortality and morbidity. Detection of co-infections elsewhere in the world [11, 12], atypical behaviour of this group of subjects in our study and response to specific treatment for the various co-infecting agents emphasises that they be considered as a separate group.
All the infections in question are arthropod borne diseases, signalling a common mode of transmission. Typically, the vectors, their breeding habits and the biting behaviours are different for different infections. The rains and water-logging during monsoons and growth of vegetation catalyse the proliferation of the vectors in general. The predisposed individuals were apparently exposed to the various vectors by virtue of their occupation or habits, leading to transmission of multiple organisms. Cross-transmission by a single vector due to hybridisation and/or mutation due to pesticide use may also have contributed. The diagnosis of co-infections also points to the low efficacy of local control measures and urbanisation [13]. Also, it seems that the traditionally rural malaria is becoming more peri-urban, and, therefore, appears to co-exist with dengue fever, a typical urban endemic disease [14].
The interaction of the malarial parasites with the host’s immune system is complex and incompletely understood. Clinical studies have suggested that P. vivax infection may be protective when co-existing with P. falciparum, with less severe disease and a lower level of parasitaemia than seen in an infection with P. falciparum alone. Also, theoretically, the response to therapy may be different in co-infections. If either of the infecting species is resistant to therapy, the other may survive to a greater degree, leading to greater parasitaemia and/or a high risk of relapse, specifically in P. vivax due to liver hypnozoites [15, 16]. In our study, P. vivax infection accounted for 39 cases, while five were co-infection of P. falciparum and P. vivax in the group with malaria. While three cases were uncomplicated, two had mild hyperbilirubinaemia consistent with the protective effect of malarial co-infection. The mortalities encountered in the group with malaria were due to infection by P. vivax and not due to P. falciparum, a point highlighted by us in a previous study from the same geographical region on the subject [10].
Co-infections appeared to reduce the occurrence of clinical features like hepato-splenomegaly seen with mono-infections of malaria and scrub typhus (p < 0.05). Even organ dysfunction was significantly less in terms of the number, severity and progression of organ dysfunction in the co-infection group as compared to mono-infections. However, this effect was not replicated when specific sub-groups of co-infections were compared with the relevant mono-infections, presumably due to the small number of subjects in each sub-group. Whether the protective or, more appropriately, the lack of summative effect is due to malaria, scrub or dengue could not be predicted and needs to be elucidated. It might be the result of some as yet unknown factors or protective antibodies elaborated in such patients as a response to co-infections. Moreover, whether malarial infection induces antibody production that cross-reacts with scrub typhus and dengue virus infection is not known. The serological profile was studied in patients with malaria and considerable cross-reactivity for IgM antibodies of other pathogens was observed in a study from south India [17]. The lack of summative (or the protective) effect might be similar to the suppression of HIV viral load in infection by scrub typhus seen in an earlier study and in vitro anti-HIV-1 in HIV-negative patients infected with scrub typhus [18]. The pathogenesis of the manifestations of scrub typhus, though still elusive, is thought to be vasculitic in nature and has the potential of being the underlying protective disease in co-infections.
Thrombocytopaenia and anaemia were more pronounced in concurrent dengue and malaria infection than single infections in the study by Epelboin et al., which was attributed to longer duration of evolution or increased virulence, in isolation or in combination [19]. In contrast, we did not observe any difference in this sub-group in the mean haemoglobin or platelet counts when compared with the mono-infections.
Pregnancy is clearly recognised as a risk factor for severe dengue virus infection leading to obstetric complications [20], but scarce literature is available on the impact of P. vivax on the course of gestation [21]. In the series by Magalhães et al., 2 of 8 female patients were pregnant and both presented with complicated disease, but no pregnancy outcome was evaluated prospectively [22]. Abortions and intra-uterine deaths occurred in 2/7, 1/3, 0/1 and 2/4 pregnant females with co-infections, malaria, dengue and scrub typhus in their second and last trimesters, respectively, in the present study. Dengue and malaria co-infection requires special attention because delayed diagnosis and inappropriate treatment may result in fatal complications [23]. The absence of fatalities in general and in pregnant females in particular and/or severe bleeding requiring blood transfusion in our study may reflect the facilitated access to diagnosis and specific treatment in this tertiary health care facility and increased awareness among the physicians. Also, there may be a difference in the serotype of the dengue virus affecting the Amazonian population [22] and our study subjects.
It might be contested that the diagnosis was mainly serological for scrub typhus and dengue, and that pooled sera were not analysed. Also, the non-utilisation of polymerase chain reaction (PCR) and/or culture for confirming the presence of an organism was another limitation. However, this facility is lacking in most of the countries bearing the brunt of these infections and, hence, our observations are important and realistic for such setups. The small number of cases in the specific co-infections was a major limitation in bringing out the differences with the relevant mono-infection, if any. Another limitation of our study was the lack of identification of the dengue virus serotype; however, the limitation was offset by comparing the features of the mono- and co-infections.
Despite the limitations, the study has been able to bring to light an unrecognised yet significant aetiological group being frequently observed in this part of the world. Studies with larger sample sizes are needed in South Asia and in other tropical regions to ratify the existence of this discrete group of co-infections and to identify the geographical and genetic variation in the variety, presentation and outcome. Whether the lack of summation of adverse effects is valid and extends to the pregnant and paediatric population needs to be addressed and is the subject of future research.
Conclusion
Acute undifferentiated febrile illness (AUFI) may be caused by a single or a combination of organisms. In contrast to the presupposition that co-infections cause severe disease and greater mortality, the presentation and severity of co-infections appears to be milder and outcomes comparable (if not better) to the mono-infections. The search for multiple aetiologies should be incorporated in the initial diagnostic workup of patients with AUFIs. This may have potential ramifications in terms of treatment and understanding the prognosis, especially in the resource-constrained tropical settings.
References
Mussini C, Pezzotti P, Miró JM et al (2004) Discontinuation of maintenance therapy for cryptococcal meningitis in patients with AIDS treated with highly active antiretroviral therapy: an international observational study. Clin Infect Dis 38:565–571
Kaufmann SH, McMichael AJ (2005) Annulling a dangerous liaison: vaccination strategies against AIDS and tuberculosis. Nat Med 11:S33–S44
Watt G, Kantipong P, de Souza M et al (2000) HIV-1 suppression during acute scrub-typhus infection. Lancet 356:475–479
Xiang J, George SL, Wünschmann S, Chang Q, Klinzman D, Stapleton JT (2004) Inhibition of HIV-1 replication by GB virus C infection through increases in RANTES, MIP-1α, MIP-1β, and SDF-1. Lancet 363:2040–2046
Kublin JG, Patnaik P, Jere CS et al (2005) Effect of Plasmodium falciparum malaria on concentration of HIV-1-RNA in the blood of adults in rural Malawi: a prospective cohort study. Lancet 365:233–240
Bradley DJ, Warrell DA (2003) Malaria. In: Warrell DA, Cox TM, Firth JD, Benz EH (eds) Oxford textbook of medicine, 4th edn. Oxford University Press, Oxford, pp 721–723
Nacher M, Gay F, Singhasivanon P et al (2000) Ascaris lumbricoides infection is associated with protection from cerebral malaria. Parasite Immunol 22:107–113
Watt G, Jongsakul K, Suttinont C (2003) Possible scrub typhus coinfections in Thai agricultural workers hospitalized with leptospirosis. Am J Trop Med Hyg 68:89–91
Sharma A, Raina R, Dhiman P, Bellad A, Madhabhavi I, Panda P (2012) Rare coinfection of scrub typhus and malaria in immunocompetent person. Online J Health Allied Sci 11(2):1–2
Srivastava S, Ahmad S, Shirazi N, Kumar Verma S, Puri P (2011) Retrospective analysis of vivax malaria patients presenting to tertiary referral centre of Uttarakhand. Acta Trop 117:82–85
Singhsilarak T, Phongtananant S, Jenjittikul M et al (2006) Possible acute coinfections in Thai malaria patients. Southeast Asian J Trop Med Public Health 37(1):1–4
Lee CH, Liu JW (2007) Short report: coinfection with leptospirosis and scrub typhus in Taiwanese patients. Am J Trop Med Hyg 77(3):525–527
Alirol E, Getaz L, Stoll B, Chappuis F, Loutan L (2011) Urbanisation and infectious diseases in a globalised world. Lancet Infect Dis 11:131–141
Ferreira Gonçalves MJ, Alecrim WD (2004) Non-planed urbanization as a contributing factor for malaria incidence in Manaus-Amazonas, Brazil. Rev Salud Publica (Bogota) 6:156–166
Mason DP, Krudsood S, Wilairatana P et al (2001) Can treatment of P. vivax lead to a unexpected appearance of falciparum malaria? Southeast Asian J Trop Med Public Health 32:57–63
Mason DP, McKenzie FE (1999) Blood-stage dynamics and clinical implications of mixed Plasmodium vivax–Plasmodium falciparum infections. Am J Trop Med Hyg 61:367–374
Mørch K, Vivek R, Xena D et al (2014) Serological profile among patients with malaria in secondary community hospitals in India—a multicenter cross sectional study. Malar J 13(Suppl 1):63
Watt G, Kantipong P, Burnouf T, Shikuma C, Philpott S (2013) Natural scrub typhus antibody suppresses HIV CXCR4(X4) viruses. Infect Dis Rep 5(e8):27–31
Epelboin L, Hanf M, Dussart P et al (2012) Is dengue and malaria co-infection more severe than single infections? A retrospective matched-pair study in French Guiana. Malar J 11:142
Thaithumyanon P, Thisyakorn U, Deerojnawong J, Innis BL (1994) Dengue infection complicated by severe hemorrhage and vertical transmission in a parturient woman. Clin Infect Dis 18:248–249
Chagas EC, do Nascimento CT, de Santana Filho FS et al (2009) Impact of malaria during pregnancy in the Amazon region. Rev Panam Salud Publica 26:203–208
Magalhães BML, Alexandre MAA, Siqueira AM et al (2012) Clinical profile of concurrent dengue fever and plasmodium vivax malaria in the Brazilian amazon: case series of 11 hospitalized patients. Am J Trop Med Hyg 87(6):1119–1124
Costa AdeP, Bressan CdaS, Pedro RS et al (2010) Delayed diagnosis of malaria in a dengue endemic area in the Brazilian extra-Amazon: recent experience of a malaria surveillance unit in state of Rio de Janeiro. Rev Soc Bras Med Trop 43:571–574
Author contributions
SA, MD, NKB: conceptualised the study
SA, MD, VK, GM, HCS, VG: collected data
SA, HCS, NS: interpreted data
SA, NKB, GM, NS: drafted the manuscript
NKB, GM, NS, VG: provided critical intellectual inputs
SA, MD, GM, NKB, NS, VK, HCS, VG: approved the manuscript
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Formal consent was not required as this was a retrospective observational study.
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
Nil.
Rights and permissions
About this article
Cite this article
Ahmad, S., Dhar, M., Mittal, G. et al. A comparative hospital-based observational study of mono- and co-infections of malaria, dengue virus and scrub typhus causing acute undifferentiated fever. Eur J Clin Microbiol Infect Dis 35, 705–711 (2016). https://doi.org/10.1007/s10096-016-2590-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2590-3