Abstract
The prevalence of multidrug-resistant (MDR) Pseudomonas aeruginosa has increased over the past decade and a significant rise in these isolates in ventilator-associated pneumonia (VAP) has been observed. However, the impact of MDR on VAP outcome has not been analysed in depth. We investigated the risk factors for early and crude mortality in a retrospective study of microbiologically and clinically documented VAP. Ninety-one VAP episodes in 83 patients were included, 31 caused by susceptible P. aeruginosa and 60 by MDR strains, of which 42 (70 %) were extensively drug-resistant (XDR) P. aeruginosa. Thirteen episodes concomitantly presented P. aeruginosa bacteraemia, in seven of which the origin was the respiratory tract. Whereas susceptible P. aeruginosa episodes were more likely than MDR episodes to receive adequate empirical (68 % vs. 30 %; p < 0.001) and definitive antimicrobial therapy (96 % vs. 50 %; p < 0.001), susceptible P. aeruginosa VAP presented a trend towards early mortality (29 % vs. 15 %; p = 0.06). A logistic regression model with early mortality as the dependent variable identified multiorgan dysfunction syndrome (MODS) [odds ratio (OR) 10.4; 95 % confidence interval (CI) 1.7–63.5; p = 0.01] and inadequate antibiotic therapy (OR 4.27; 95 % CI 0.98–18.4; p = 0.052) as independent risk factors for early mortality. A similar analysis identified MODS (OR 4.31; 95 % CI 1.14–16.2; p = 0.03) as the only independent predictor of crude mortality. The severity of acute illness clinical presentation was the main predictor of mortality. Despite adequate antibiotic therapy, susceptible P. aeruginosa seems to cause major early mortality. Although adequate therapy is essential to treat VAP, the severity of acute illness is a more important factor than drug resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intubation with mechanical ventilation increases the risk of lower respiratory tract infections, and is associated with crude mortality rates of 30–70 % [1]. Pseudomonas aeruginosa is one of the main species implicated in the pathogenesis of ventilator-associated pneumonia (VAP), and the emergence of P. aeruginosa strains which exhibit co-resistance to almost all antibiotic classes is growing. The presence of multidrug-resistant (MDR) strains diminishes the treatment options and increases the risk of inadequate empirical therapy.
Guidelines for the management [1] of VAP emphasised the importance of early, adequate, empirical antibiotic therapy based on patient risk factors for infection due to MDR pathogens. Potential disadvantages include excessive use of multiple empirical antibiotic therapy, which may increase antibiotic resistance, toxicity and failure to properly de-escalate when culture data become unavailable.
Multiple antibiotic therapy is commonly administered in cases of suspected or proven P. aeruginosa infections, especially hospital-acquired pneumonia (HAP). However, this strategy remains controversial, since the administration of one effective antibiotic therapy achieves similar mortality rates [2, 3]. In fact, it is well known that mortality in these patients is influenced at least as much by their underlying condition as by the organism itself [4, 5]. Several studies have shown an increase in mortality with a delay in adequate antibiotics [6–8], but others have failed to find a significant difference in mortality when patients received inadequate therapy [9–11]. Furthermore, a recent prospective study [12] suggested that the deleterious effect of the antimicrobial resistance in P. aeruginosa may be not as great during the first days of the bacteraemia. It is very important to clarify prognostic factors related to antimicrobial resistance because they may help guide the recommended empirical antibiotic policy in the setting of MDR P. aeruginosa. If a delay in adequate antibiotic therapy does not have decisive consequences in most of these patients, then the indiscriminate use of empirical colistin could be avoided, along with the consequent risk of increasing resistance to this drug.
The prevalence of MDR P. aeruginosa has increased over the past decade. Nonetheless, and despite a significant rise in these MDR P. aeruginosa isolates in VAP infections, few studies of this issue have been published. While the risk factors for MDR organisms [13, 14] or antibiotic treatment of VAP [15] have aroused the interest of clinicians, to our knowledge, few studies have quantified the effect of MDR P. aeruginosa, and the possible effect of delaying adequate antimicrobial treatment, on mortality.
A progressive increase in the number of MDR P. aeruginosa isolates has been observed in our hospital in an epidemiological setting of a sustained endemic outbreak [16]. We designed a retrospective study among patients with ventilator-associated lower respiratory tract infections, with the main objective of investigating the risk factors for early and crude mortality. We also analysed the impact of multidrug resistance on VAP outcome and compared the epidemiological characteristics of VAP caused by susceptible P. aeruginosa and those caused by MDR P. aeruginosa.
Materials and methods
Study design
A retrospective study was performed at Hospital de Bellvitge, a tertiary-care university hospital between January 2006 and December 2011. All patients selected met the following criteria: positive results of quantitative culture of specimens from bronchoalveolar lavage (BAL) and microbiologic confirmation of quantitative monomicrobial cultures with a P. aeruginosa bacterial count of 1 × 105 colony-forming units (CFU)/mL in BAL fluid specimen [1]. HAP was defined according to the Clinical Pulmonary Infection Score (CPIS) [17] and a score ≥6, indicating the presence of respiratory tract infection, was the condition for inclusion.
The following data were recorded: age and sex; comorbidities (diabetes mellitus, chronic obstructive pulmonary disease, heart failure, chronic renal failure, hepatic dysfunction, haematologic and solid malignancy, neurologic disease and immune deficiency disease) and severity of underlying diseases calculated using the Charlson comorbidity index [18]; the presence of neutropaenia (absolute granulocyte count of <500/ml) and the use of immunosuppressive therapy (chemotherapy, radiotherapy and/or immunosuppressive drugs) during the 30 days prior to VAP presentation; the presence of septic shock, multiorgan dysfunction syndrome (MODS) at VAP presentation [19]; and antimicrobial treatment received. The severity of illness was estimated by the Simplified Acute Physiology Score (SAPS II) at intensive care unit (ICU) admission and at sample collection [20]. The length of hospitalisation prior to the collection of P. aeruginosa culture sample and mechanical ventilation at the time of the culture sample were also recorded.
Definitions
HAP is defined as the development of parenchymal lung infection after at least 48 h of hospitalisation. The infection develops after the patient has undergone intubation and received mechanical ventilation for at least 48 h; the condition is termed VAP. Ventilator-associated tracheobronchitis (VAT) is a clinical syndrome similar to VAP, but with no radiographic infiltrate present [21]. VAP and VAT were established according to clinical diagnosis and the collection of a deep respiratory tract sample for culture in order to establish an aetiologic pathogen. The diagnosis of HAP is made in the case of a CPIS ≥6 indicating the presence of respiratory tract infection based on criteria of fever, elevated white blood cell count, purulent appearance of respiratory secretions, radiographic findings and growth of pathogens on a lower respiratory tract culture [17]. Bacteraemia of origin other than the respiratory tract was considered when it occurred more than 72 h after VAP diagnosis.
Delay in adequate antimicrobial therapy was defined as the time from VAP until administration of the empirical or definitive adequate therapy. Empirical therapy was considered when an antimicrobial regimen was administered within 24 h of VAP diagnosis and before susceptibility was known; therapy administered after this time was considered to be definitive. Antimicrobial therapy was considered adequate when the P. aeruginosa isolate was susceptible to the antimicrobial prescribed. Combination therapy was defined as adequate if the strain was susceptible to both antipseudomonal drugs given simultaneously. Aminoglycoside monotherapy and inhaled colistin alone were considered inadequate treatment for P. aeruginosa VAP. In addition, piperacillin–tazobactam treatment was considered inadequate when the strain showed a minimum inhibitory concentration (MIC) in the range 32–64 mg/L in accordance with the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria [22] and the new breakpoints established by the Clinical and Laboratory Standards Institute (CLSI) [23].
Clinical outcome was classified as follows: (i) clinical cure: resolution of presenting symptoms and signs of infection by the end of antibiotic treatment; (ii) clinical improvement: partial resolution of presenting symptoms and signs of infection; (iii) clinical failure: persistence or worsening of presenting symptoms and signs of infection during the treatment; and (iv) recurrence of infection: occurrence of a new episode of infection at least 72 h after the clinical resolution of a preceding episode.
Microbiological outcome was classified as: (i) eradication: no growth of the pathogen in the final culture of specimens during the entire hospitalisation; (ii) persistence of pathogen: persistent growth of the responsible pathogen regardless of the clinical outcome of the infection; and (ii) recurrence of the pathogen: regrowth of the same pathogen regardless of the clinical outcome of the infection.
Early mortality was defined as death within the 7 days of VAP onset; crude mortality was considered as death during the hospitalisation.
Microbiological data
Microbiological data included all positive P. aeruginosa BAL and blood samples. Microbiological data on other organisms causing bloodstream infections at a distal site were also documented. P. aeruginosa strains were identified and tested for antimicrobial susceptibility by a MicroScan automated microdilution system using CN1S and CO1S panels (Dade International, West Sacramento, CA, USA). CLSI criteria [23] were used to define susceptibility or resistance to these antimicrobial agents. The antibiotics tested were: piperacillin–tazobactam, ceftazidime, cefepime, aztreonam, imipenem, meropenem, ciprofloxacin, ofloxacin, gentamicin, tobramycin, amikacin, colistin and fosfomycin. Intermediate results were considered to be resistant.
In accordance with recent standard definitions [24], MDR P. aeruginosa was defined as a strain non-susceptible to ≥1 agent in ≥3 antipseudomonal antimicrobial categories. Extensively drug-resistant (XDR) P. aeruginosa was defined as non-susceptible to ≥1 agent in all but ≤2 antipseudomonal antimicrobial categories; thus, an XDR P. aeruginosa isolate was also included as MDR P. aeruginosa. All other P. aeruginosa isolates, including those non-susceptible to ≥1 agent in <3 antimicrobial categories, were considered to be non-MDR strains. MDR and XDR P. aeruginosa were defined considering the previously indicated agents.
Statistical analysis
Student’s t-test was used to compare continuous variables. The Chi-square test or Fisher’s exact test was used to compare categorical variables. Logistic regression analysis was used to identify the independent risk factors for VAP P. aeruginosa outcome. Variables with a p < 0.05 in the univariate analysis and those found in previous studies were included in the multivariate model. Data were analysed using the statistical software SPSS, version 15.0 (SPSS, Chicago, IL, USA).
Results
Epidemiological and clinical features
We identified 120 episodes of ventilator-associated lower respiratory tract infections for a microbiologically documented P. aeruginosa infection during the study period. Of these episodes, 29 were excluded from further analysis because they did not have a clinical lower respiratory tract infection according to the CPIS. Ninety-one episodes in 83 patients with a mean of CPIS of 8.96 (range 6 to 14) were analysed. Six patients experienced a second VAP episode and one a third episode. There were four recurrences of the initial VAP, all caused by XDR P. aeruginosa strains; in the remaining four episodes, the initial and subsequent P. aeruginosa strains isolated from the respiratory tract from the same patients showed different susceptibility profiles; the intervals between the first and second episodes were 21, 18, 29 and 58 days, respectively. Three patients were classified as VAT; here, we use the terms VAP and ventilator-associated respiratory tract infection interchangeably.
Of the 83 patients, 65 (78 %) were male and the mean age was 69.39 ± 14.15 years. The mean length of ICU admission to VAP was 24.93 ± 22.0 days and the mean time from mechanical ventilation to VAP was 19.06 ± 18.1 days.
Among the 91 VAP episodes, 60 were caused by MDR P. aeruginosa, of which 42 (70 %) were XDR strains. Thirteen VAP episodes concomitantly presented P. aeruginosa bacteraemia; in seven, the source was the respiratory tract [7] and the remaining six were primary bacteraemias (catheter vascular and unknown origin). Five other bloodstream infections were detected during the VAP clinical evolution: two Klebsiella pneumoniae episodes, one Escherichia coli, one Enterococcus faecalis and one E. faecium.
Among the 60 MDR episodes, 11 received intravenous (i.v.) colistin. In all but one, the isolates of MDR P. aeruginosa from respiratory specimens were due to XDR strains. Four of the 11 patients survived and in two, the dosage of colistin was adequate; the remaining seven patients died and only two patients received adequate doses of i.v. colistin. None of these deaths occurred within 7 days of VAP onset. In addition, inhaled colistin was administered in 48 (80 %) MDR episodes.
Thirty (50 %) of the MDR P. aeruginosa episodes received inadequate definitive therapy, 24 (80 %) of which were caused by XDR strains. Ten XDR episodes received piperacillin–tazobactam, according to the prior CLSI susceptibility range; nine received antimicrobials (three carbapenems, four antipseudomonal cephalosporins, two fluoroquinolones) with intermediate range of activity; and the remaining five episodes received inhaled colistin alone.
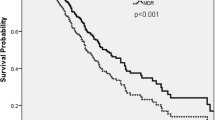
The epidemiological and clinical characteristics of the VAP episodes according to the susceptibility of the P. aeruginosa strain are shown in Table 1. Among the 91 VAP episodes, the median days to ICU admission from VAP onset was significantly higher in patients with MDR strains, as was the interval of days between the start of mechanical ventilation and VAP diagnosis. Patients in the non-MDR strain VAP group were more likely than those in the MDR group to receive adequate empirical (68 % vs. 30 %; p < 0.001) and definitive antimicrobial therapy (96 % vs. 50 %; p < 0.001). We also observed a trend towards early mortality in susceptible P. aeruginosa VAP (29 % vs. 15 %; p = 0.06). The microbiological outcome was better in non-MDR episodes but did not reach statistical significance.
Outcome
Early mortality
Eighteen patients died during the 7 days after VAP onset. The unadjusted analyses for the association of the characteristics with mortality are shown in Table 2. A logistic regression model with early mortality as a dependent variable, adjusted for age, sex, severity of illness, bacteremic VAP, shock, MODS and adequate empirical antibiotic treatment, identified MODS [odds ratio (OR) 10.4; 95 % confidence interval (CI) 1.7–63.5; p = 0.011] and adequate antibiotic treatment (OR 4.27; 95 % CI 0.98–18.4; p = 0.052) as independent risk factors for early mortality.
Crude mortality
Forty-seven patients died during hospitalisation [17 (55 %) susceptible group vs. 30 (50 %) MDR group; p = 0.33] (Table 3). Severity of acute illness and clinical presentation were the variables associated with crude mortality. We also observed a trend towards an association between crude mortality and severity of illness at ICU admission (p = 0.06). A logistic regression model with crude mortality as the dependent variable, adjusted for age, sex, severity of illness, shock and MODS, identified MODS (OR 4.31; 95 % CI 1.14–16.2; p = 0.03) as the only independent risk factor for crude mortality. Adjusted analysis with adequate empirical or definitive antimicrobial therapy did not modify the model.
Discussion
Multiple reports over the last decade have alerted on the increase of MDR P. aeruginosa strains in hospitals worldwide, mainly in ICUs. In this study, MDR P. aeruginosa was frequently identified, with an overall prevalence in respiratory tract infections of 66 %. This high prevalence may be attributed to the epidemiological problem observed in our hospital during the study period [16].
In this series, VAP caused by MDR P. aeruginosa had longer duration of mechanical ventilation, longer ICU stay, higher rate of underlying disease and a longer delay in adequate antimicrobial therapy administration, but it was not followed by significant differences in clinical response and crude mortality. In fact, the impact of drug resistance on the outcome of VAP remains controversial; some [6, 25], but not all, studies [26 27] find a relationship between poorer clinical outcome and the presence of resistant organisms.
Conversely, a trend towards early mortality linked to susceptible P. aeruginosa respiratory tract infections (29 % vs. 15 %; p = 0.06) was observed. In fact, empirical antimicrobial therapy and treatment in the first 24 h were seen to be adequate in VAP episodes with early mortality (67 % vs. 36 %), suggesting that patients who have comorbidities and are severely ill at the time of VAP diagnosis are at a high risk of treatment failure, despite receiving adequate antibiotic therapy [28]. A plausible explanation for the association between susceptible strains and worse early mortality is that, hypothetically, they are more virulent than their drug-resistant counterparts [29]. Although we did not observe significant differences in clinical presentation between the two phenotype groups, we cannot rule out the possibility that unmeasured factors predispose to a major deleterious effect in susceptible strains.
The crude mortality rate for HAP may be as high as 70 %, but many of these critically ill patients with HAP die of their underlying disease rather than from pneumonia [4]. In our study, MODS clinical presentation was the only predictor for crude mortality. In fact, previous clinical studies have identified severity of illness at the moment of diagnosis or the development of shock or/and MODS during clinical presentation as key prognostic factors [30, 31]. Thus, our results do not support the idea that VAP caused by MDR P. aeruginosa is associated with worse clinical outcome due to inadequate antimicrobial therapy. While these findings challenge those of certain studies [6–8], surprisingly, other reports [9–11] also failed to find any difference in mortality when evaluating this variable in a group of VAP due to P. aeruginosa strains susceptible only to colistin.
Regarding definitive treatment, several aspects of our series should be noted. A high number of our patients, indeed, received inadequate definitive treatment, because around 50 % of MDR episodes were treated with inhaled colistin and piperacillin–tazobactam, which are currently considered to be inadequate, but were within the susceptible range according to earlier CLSI criteria. Similarly, although the efficacy of colistin has been reported in several series of pneumonia [15], intravenous colistin was administered in only 11 (18 %) VAP episodes. In addition, according to pharmacokinetics/pharmacodynamics studies, the dosage regimens were suboptimal in all but four episodes; moreover, aerosolised colistin was added as adjunctive therapy to other antimicrobials for the treatment of VAP in 80 % of the MDR episodes. The effectiveness of inhaled colistin as monotherapy in 11 of the 21 XDR episodes who survived may be indicative of the clinical benefit of this mode of administration.
Despite the high prevalence of MDR and XDR strains in our hospital, these data merely reflect the fact that colistin was not considered as the first-line therapy, and that a delayed onset of colistin treatment did not entail worse prognosis. These findings could be useful to strengthen the design of empirical antibiotic strategies, reducing colistin consumption and, therefore, reducing the risk of developing resistance. It seems advisable to reserve colistin for use as definitive therapy in critical patients with VAP due to MDR and XDR P. aeruginosa isolates.
The present study has the inherent limitations of a retrospective analysis and the sample size is rather small for some comparisons. However, to evaluate the predictors for mortality, we adjusted for confounding variables such as severity of illness at the moment of diagnosis, severity of clinical presentation or the presence of bacteraemia, factors which were associated with fatality in previous studies. SAPS II provides outcome prediction scores that share many variables in their calculation, and the use of this score and the severity of clinical presentation (shock and MODS) in the same model may cause collinearity problems in the interpretation of the results. Due to this potential collinearity problem, and after repeating the analysis with different combinations, only shock and MODS are included in the final analysis. Finally, the inferences that can be made from our data may be limited, since previous data indicate clonal involvement in the XDR phenotype [16]; moreover, a recent study [12] showed that the major XDR clone involved in our hospital was widely disseminated in Spanish hospitals and, likely, other countries. Thus, we believe that these results are applicable to other settings.
In conclusion, this is one of the largest series of monomicrobial P. aeruginosa VAP reported to date, with a high prevalence of MDR strains. We found that multidrug resistance does not adversely affect the outcome for patients with VAP. While the adequacy of empirical antimicrobial therapy is, of course, very important in the early outcome of patients with VAP, episodes caused by susceptible P. aeruginosa strains seem to have major early mortality, despite more frequently receiving adequate empirical therapy. This information on the implications of resistance for patient outcome suggests that it may be wiser to focus on improving the haemodynamic support sepsis, and may also guide antibiotic policy by allowing a more judicious use of the few antimicrobial options available. Finally, although adequate therapy is essential to treat VAP infection, it seems that the severity of acute illness is more important than resistance.
References
Niederman MS, Craven DE, Bonten MJ, Chastre J, Craig WA, Fagon JY et al (2005) Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 171:388–416
Eggimann P, Revelly JP (2006) Should antibiotic combinations be used to treat ventilator-associated pneumonia? Semin Respir Crit Care Med 27:68–81
Garnacho-Montero J, Sa-Borges M, Sole-Violan J, Barcenilla F, Escoresca-Ortega A, Ochoa M et al (2007) Optimal management therapy for Pseudomonas aeruginosa ventilator-associated pneumonia: an observational, multicenter study comparing monotherapy with combination antibiotic therapy. Crit Care Med 35:1888–1895
Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun-Buisson C; Canadian Critical Trials Group (1999) The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. Am J Resp Crit Care Med 159:1249–1256
Gursel G, Demirtas S (2006) Value of APACHE II, SOFA and CPIS scores in predicting prognosis in patients with ventilator-associated pneumonia. Respiration 73:503–508
Kollef MH, Silver P, Murphy DM, Trovillion E (1995) The effect of late-onset ventilator-associated pneumonia in determining patient mortality. Chest 106:1655–1662
Luna CM, Videla A, Mattera J, Vay C, Famiglietti A, Vujacich P et al (1999) Blood cultures have limited value in predicting severity of illness and as a diagnostic tool in ventilator-associated pneumonia. Chest 116:1075–1084
Luna CM, Aruj P, Niederman MS, Garzón J, Violi D, Prignoni A et al (2006) Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. Eur Respir J 27:158–164
Garnacho-Montero J, Ortiz-Leyba C, Jiménez-Jiménez FJ, Barrero-Almodóvar AE, García-Garmendia JL, Bernabeu-Wittell M et al (2003) Treatment of multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia (VAP) with intravenous colistin: a comparison with imipenem-susceptible VAP. Clin Infect Dis 36:1111–1118
Reina R, Estenssoro E, Sáenz G, Canales HS, Gonzalvo R, Vidal G et al (2005) Safety and efficacy of colistin in Acinetobacter and Pseudomonas infections: a prospective cohort study. Intensive Care Med 31:1058–1065
Rios FG, Luna CM, Maskin B, Saenz Valiente A, Lloria M, Gando S et al (2007) Ventilator-associated pneumonia due to colistin susceptible-only microorganisms. Eur Respir J 30:307–313
Peña C, Suarez C, Gozalo M, Murillas J, Almirante B, Pomar V et al; Spanish Network for research in Infectious Diseases (REIPI) (2012) Prospective multicenter study of the impact of carbapenem resistance on mortality in Pseudomonas aeruginosa bloodstream infections. Antimicrob Agents Chemother 56:1256–1272
Lodise TP, Miller CD, Graves J, Furuno JP, McGregor JC, Lomaestro B et al (2007) Clinical prediction tool to identify patients with Pseudomonas aeruginosa respiratory tract infections at greatest risk for multidrug resistance. Antimicrob Agents Chemother 51:417–422
Parker CM, Kutsogiannis J, Muscedere J, Cook D, Dodek P, Day AG et al; Canadian Critical Care Trials Group (2008) Ventilator-associated pneumonia caused by multidrug-resistant organisms or Pseudomonas aeruginosa: prevalence, incidence, risk factors, and outcomes. J Crit Care 23:18–26
Florescu DF, Qiu F, McCartan MA, Mindru C, Fey PD, Kalil AC (2012) What is the efficacy and safety of colistin for the treatment of ventilator-associated pneumonia? A systematic review and meta-regression. Clin Infect Dis 54:670–680
Suarez C, Peña C, Arch O, Dominguez MA, Tubau F, Juan C et al (2011) A large sustained endemic outbreak of multiresistant Pseudomonas aeruginosa: a new epidemiological scenario for nosocomial acquisition. BMC Infect Dis 11:272
Pugin J, Auckenthaler R, Mili N, Janssens JP, Lew PD, Suter PM (1991) Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis 143:1121–1129
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383
American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20:864–874
Le Gall JR, Loirat P, Alperovitch A, Glaser P, Granthil C, Mathieu D et al (1984) A simplified acute physiology score for ICU patients. Crit Care Med 12:975–977
Niederman MS (2010) Hospital-acquired pneumonia, health care-associated pneumonia, ventilator-associated pneumonia, and ventilator-associated tracheobronchitis: definitions and challenges in trial design. Clin Infect Dis 51(Suppl 1):S12–S17
European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters (2008) [updated 27 April, 2010; last accessed 29 October, 2010]. Available online at: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/EUCAST_breakpoints_v1.1
Clinical and Laboratory Standards Institute (CLSI) (2012) Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Second Informational Supplement. CLSI document M100-S22. CLSI, Wayne, PA
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG et al (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281
Combes A, Luyt CE, Fagon JY, Wolff M, Trouillet JL, Chastre J (2007) Early predictors for infection recurrence and death in patients with ventilator-associated pneumonia. Crit Care Med 35:146–154
Combes A, Luyt CE, Fagon JY, Wollf M, Trouillet JL, Gibert C et al; PNEUMA Trial Group (2004) Impact of methicillin resistance on outcome of Staphylococcus aureus ventilator-associated pneumonia. Am J Respir Crit Care Med 170:786–792
Combes A, Luyt CE, Fagon JY, Wolff M, Trouillet JL, Chastre J (2006) Impact of piperacillin resistance on the outcome of Pseudomonas ventilator-associated pneumonia. Intensive Care Med 32:1970–1978
Gursel G, Aydogdu M, Ozyilmaz E, Ozis TN (2008) Risk factors for treatment failure in patients with ventilator-associated pneumonia receiving appropriate antibiotic therapy. J Crit Care 23:34–40
Deptuła A, Gospodarek E (2010) Reduced expression of virulence factors in multidrug-resistant Pseudomonas aeruginosa strains. Arch Microbiol 192:79–84
Dupont H, Montravers P, Gauzit R, Veber B, Pouriat JL, Martin C; Club d’Infectiologie en Anesthésie-Réanimation (2003) Outcome of postoperative pneumonia in the Eole study. Intensive Care Med 29:179–188
Garnacho-Montero J, Ortiz-Leyba C, Fernández-Hinojosa E, Aldabó-Pallás T, Cayuela A, Márquez-Vácaro JA et al (2005) Acinetobacter baumannii ventilator-associated pneumonia: epidemiological and clinical findings. Intensive Care Med 31:649–655
Acknowledgements
This work was supported by National Health Service grants FIS 08/0276 and FIS11/0164 from the Fondo de Investigación Sanitaria and was also supported by Ministerio de Ciencia e Innovación, Instituto de Salud Carlos III and Ciber de Enfermedades Respiratorias (CB06/06/0037).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peña, C., Gómez-Zorrilla, S., Oriol, I. et al. Impact of multidrug resistance on Pseudomonas aeruginosa ventilator-associated pneumonia outcome: predictors of early and crude mortality. Eur J Clin Microbiol Infect Dis 32, 413–420 (2013). https://doi.org/10.1007/s10096-012-1758-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-012-1758-8