Abstract
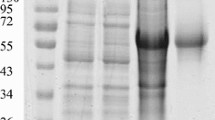
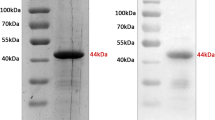
We have developed a specific reverse ELISA to investigate the potential of the domain III of the West Nile virus envelope protein to detect virus-specific antibodies. For this purpose, the domain III antigen was expressed in Escherichia coli, refolded and directly labelled with peroxidase. By mixing serum samples with the enzyme-labelled antigen, immune complexes will form that are simultaneously recognized by rheumatoid factor coated microtiter plates. Specific antibodies to the domain III were found in 160 out of 206 sera of patients with neutralising antibodies to West Nile virus (77.6% sensitivity). The antigen differentiated reliably between sera of West Nile virus- and dengue virus-infected patients. In combination with indirect immunofluorescence, to exclude four false-positive samples, the specificity was 100% (280 samples). Assuming a prevalence rate of anti-West-Nile virus antibodies of 5%, the positive and negative predictive values were 93.3% and 98.8% respectively. These results indicate that the reverse ELISA using a specific portion of the envelope antigen of West Nile virus is especially suitable for studies on the prevalence of West Nile infections in affected countries.



Similar content being viewed by others
References
CDC (1999) Outbreak of West Nile-like viral encephalitis-New York 1999. MMWR Morb Mortal Wkly Report 48
Johnson AJ, Noga AJ, Kosoy O, Lanciotti RS, Johnson AA, Biggerstaff BJ (2005) Duplex microsphere-based immunoassay for detection of anti-West Nile virus and anti-St. Louis encephalitis virus immunoglobulin m antibodies. Clin Diagn Lab Immunol 12(5):566–574
Tardei G, Ruta S, Chitu V, Rossi C, Tsai TF, Cernescu C (2000) Evaluation of immunoglobulin M (IgM) and IgG enzyme immunoassays in serologic diagnosis of West Nile Virus infection J Clin Microbiol 38(6):2232–2239
Roehrig JT (2003) Antigenic structure of flavivirus proteins. Adv Virus Res 59:141–175
Wong SJ, Demarest VL, Boyle RH, Wang T, Ledizet M, Kar K, Kramer LD, Fikrig E, Koski RA (2004) Detection of human anti-flavivirus antibodies with a West Nile virus recombinant antigen microsphere immunoassay. J Clin Microbiol 42(1):65–72
Niedrig M, Vaisviliene D, Teichmann A, Klockmann U, Biel SS (2001) Comparison of six different commercial IgG-ELISA kits for the detection of TBEV-antibodies. J Clin Virol 20(3):179–182
Hayes EB, Gubler DJ (2006) West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med 57:181–194
Heinz FX, Berger R, Tuma W, Kunz C (1983) A topological and functional model of epitopes on the structural glycoprotein of tick-borne encephalitis virus defined by monoclonal antibodies. Virology 126(2):525–537
Guirakhoo F, Heinz FX, Kunz C (1989) Epitope model of tick-borne encephalitis virus envelope glycoprotein E: analysis of structural properties, role of carbohydrate side chain, and conformational changes occurring at acidic pH. Virology 169(1):90–99
Mandl CW, Guirakhoo F, Holzmann F, Heinz FX, Kunz C (1989) Antigenic structure of the flavivirus envelope protein E at the molecular level, using tick-borne encephalitis virus as a model. J Virol 63(2):564–571
Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC (1995) The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 375(6529):291–298
Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukopadhyay S, Chipman PR, Strauss EG, Bakers TS, Strauss JH (2002) Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108(5):717–725
Fonseca BA, Khoshnood K, Shope RE, Mason PW (1991) Flavivirus type-specific antigens produced from fusions of a portion of the E protein gene with the Escherichia coli trpE gene. Am J Trop Med Hyg 44(5):500–508
Simmons M, Porter KR, Escamilla J, Graham R, Watts DM, Eckels KH, Hayes CG (1998) Evaluation of recombinant dengue viral envelope B domain protein antigens for the detection of dengue complex-specific antibodies. Am J Trop Med Hyg 58(2):144–151
Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, Ebel GD, Kramer LD, Fremeont DH, Diamond MS (2005) Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med 11(5):522–530
Ludolfs D, Schilling S, Altenschmidt J, Schmitz H (2002) Serological differentiation of infections with dengue virus serotypes 1 to 4 by using recombinant antigens. J Clin Microbiol 40(11):4317–4320
Beasley DW, Holbrook MR, Travassos da Rosa AP, Coffey L, Carrara AS, Phillippi-Falkenstein K, Bohm RP Jr, Ratterree MS, Lillibridge KM, Ludwig GV, Estrada-Franco J, Weaver SC, Tesh RB, Shope RE, Barrett AD (2004) Use of a recombinant envelope protein subunit antigen for specific serological diagnosis of West Nile virus infection. J Clin Microbiol 42(6):2759–2765
Johnson AJ, Martin DA, Karabatsos N, Roehrig JT (2000) Detection of anti-arboviral immunoglobulin G by using a monoclonal antibody-based capture enzyme-linked immunosorbent assay. J Clin Microbiol 38(5):1827–1831
Miyazawa H, Bannai H, Yanase T, Morita C, Satoh S, Sugiyama J, Tanaguchi S, Inouye S (1999) A reverse-sandwich enzyme-linked immunosorbent assay for verocytotoxin 1 and 2 antibodies in human and bovine sera. Clin Diagn Lab Immunol 6(5):701–704
Sachers M, Emmerich P, Mohr H, Schmitz H (1985) Simple detection of antibodies to different viruses using rheumatoid factor and enzyme-labelled antigen (ELA). J Virol Methods 10(2):99–110
Emmerich P, Thome-Bolduan C, Drosten C, Gunther S, Ban E, Sawinsky I, Schmitz H (2006) Reverse ELISA for IgG and IgM antibodies to detect Lassa virus infections in Africa. J Clin Virol 37(4):277–281
Schmitz H, von Deimling U, Flehmig B (1980) Detection of IgM antibodies to cytomegalovirus (CMV) using an enzyme-labelled antigen (ELA). J Gen Virol 50(1):59–68
McIntosh BM, Jupp PG, Dos Santos IS, Meenehan GM (1976) Culex (Eumelanomyia) rubinotus Theobald as vector of Banzi, Germiston and Witwatersand viruses. I. Isolation of virus from wild populations of C. rubinotus. J Med Entomol 12(6):637–640
Jupp PG, Blackburn NK, Thompson DL, Meenehan, GM (1986) Sindbis and West Nile virus infections in the Witwatersrand-Pretoria region. S Afr Med J 70(4):218–220
Schilling S, Ludolfs D, Van An L, Schmitz H (2004) Laboratory diagnosis of primary and secondary dengue infection. J Clin Virol 31(3):179–184
Stich A, Gunther S, Drosten C, Emmerich P, Dwyer DE, Hueston L, Hetzel W, Kirschner A, Fleischer K (2003) Clinical and laboratory findings on the first imported case of Murray Valley encephalitis in Europe. Clin Infect Dis 37(2):e19–21
Laue T, Emmerich P, Schmitz H (1999) Detection of dengue virus RNA in patients after primary or secondary dengue infection by using the TaqMan automated amplification system. J Clin Microbiol 37(8):2543–2547
Swanepoel R , Struthers JK, Erasmus MJ, Shepherd SP, McGillivray GM, Erasmus BJ, Barnard BJ (1986) Comparison of techniques for demonstrating antibodies to Rift Valley fever virus. J Hyg (Lond) 97(2):317–329
Avrameas S (1975) Studies on antibody formation with enzyme markers. Ann N Y Acad Sci 254:175–189
Wengler G, Wengler G (1989) An analysis of the antibody response against West Nile virus E protein purified by SDS-PAGE indicates that this protein does not contain sequential epitopes for efficient induction of neutralising antibodies. J Gen Virol 70(Pt 4):987–992
Kuno G, Gubler DJ, Oliver A (1993) Use of ’original antigenic sin’ theory to determine the serotypes of previous dengue infections. Trans R Soc Trop Med Hyg 87(1):103–105
Acknowledgments
The authors would like to thank Jan ter Meulen, Crucell, Leiden, The Netherlands and Christa Pfleiderer, Paul Ehrlich Institute, Langen, Germany for providing serum samples for the validation. We also thank Inga Nehlmeier from the Robert Koch Institute, Berlin, Germany and Stefan Linckh, Bernhard Nocht Institute Hamburg for technical assistance.
This study was supported by the Bundesministerium für Gesundheit, grant no. 1368–544, “Prävalenz und Inzidenz von West-Nil-Virus in Deutschland”. We declare that the experiments comply with the currents laws of the country in which they were performed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ludolfs, D., Niedrig, M., Paweska, J.T. et al. Reverse ELISA for the detection of anti West Nile virus IgG antibodies in humans. Eur J Clin Microbiol Infect Dis 26, 467–473 (2007). https://doi.org/10.1007/s10096-007-0309-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-007-0309-1