Abstract
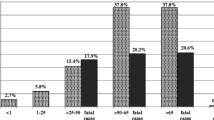
The incidence of vancomycin-resistant Enterococcus faecium isolation was low (≤5%) in German hospitals before 2003. Within the second half of 2003 and the first half of 2004, however, increasing frequencies of up to 14% were noticed in several hospitals in southwestern Germany. This increase was attributed mainly to the occurrence and spread of epidemic-virulent ampicillin/vancomycin-resistant, vanA- and vanB-positive E. faecium clones, most of which exhibited the virulence factors enterococcal surface protein (esp) and bacteriocin activity and some which exhibited hyaluronidase (hyl). E. faecium possessing hyaluronidase was initially found in U.S. hospitals and recently detected in several European hospitals and, subsequently, in German hospitals as well. Ampicillin/vancomycin-resistant E. faecium clones originating mainly from southwestern German hospitals were characterized by multilocus sequence typing since different sequence types (STs) belonging to the clonal complex-17 are currently disseminated worldwide. Multilocus sequence typing revealed that, in 1998 and 1999, ampicillin/vancomycin-resistant E. faecium clone ST-117 was prevalent in various German hospitals, while in 2003 and 2004, clone ST-203 dominated in several hospitals located in southwestern Germany. Both sequence types display single-locus variants of ST-78, which was frequently recorded in various Italian hospitals between 2000 and 2003, and all of these STs belong to the clonal complex-17. Expression of linezolid resistance was observed in ampicillin/glycopeptide-resistant E. faecium strains (VanA type) from two tertiary hospitals in southwestern Germany due to mutations in domain V of the 23S rDNA (G2576T). While in one hospital the resistance emerged during linezolid therapy, in the other hospital resistance was caused by transfer of an identical linezolid/ampicillin/glycopeptide-resistant E. faecium strain. In conclusion, it is very important to monitor the occurrence of epidemic-virulent clonal complex-17 strains of E. faecium to prevent their spread in hospitals, especially if they are resistant to glycopeptides and linezolid.

Similar content being viewed by others
References
Woodford N, Johnson AP, Morrison D, Speller DC (1995) Current perspectives on glycopeptide resistance. Clin Microbiol Rev 8:585–615
Cetinkaya Y, Falk P, Mayhall CG (2000) Vancomycin-resistant enterococci. Clin Microbiol Rev 13:686–707
Elsner HA, Sobottka I, Feucht HH, Harps E, Haun C, Mack D, Ganschow R, Laufs R, Kaulfers PM (2000) Nosocomial outbreak of vancomycin-resistant Enterococcus faecium at a German university pediatric hospital. Int J Hyg Environ Health 203:147–152
Hayden MK (2000) Insights into the epidemiology and control of infection with vancomycin-resistant enterococci. Clin Infect Dis 31:1058–1065
Bonten MJ, Willems R, Weinstein RA (2001) Vancomycin-resistant enterococci: why are they here, and where do they come from? Lancet Infect Dis 1:314–325
Gold HS (2001) Vancomycin-resistant enterococci: mechanisms and clinical observations. Clin Infect Dis 33:210–219
Borgmann S, Niklas DM, Klare I, Zabel LT, Buchenau P, Autenrieth IB, Heeg P (2004) Two episodes of vancomycin-resistant Enterococcus faecium outbreaks caused by two genetically different clones in a newborn intensive care unit. Int J Hyg Environ Health 207:386–389
Martone WJ (1998) Spread of vancomycin-resistant enterococci: why did it happen in the United States? Infect Control Hosp Epidemiol 19:539–545
Centers for Disease Control and Prevention (1993) Nosocomial enterococci resistant to vancomycin—United States, 1989–1993. Morbid Mortal Wkl Rep 42:597–599
Jones ME, Draghi DC, Thornsberry C, Karlowsky JA, Sahm DF, Wenzel RP (2004) Emerging resistance among bacterial pathogens in the intensive care unit—a European and North American surveillance study (2000–2002). Ann Clin Microbiol Antimicrob 3:14–24
Diekema DJ, Boots Miller BJ, Vaughn TE, Woolson RF, Yankey JW, Ernst EJ, Flach SD, Ward MM, Franciscus CL, Pfaller MA, Doebbeling BN (2004) Antimicrobial resistance trends and outbreak frequency in United States hospitals. Clin Infect Dis 38:78–85
Kresken M, Hafner D, Studiengruppe (2000) Resistenzsituation bei klinisch wichtigen Infektionserregern gegenüber Chemotherapeutika in Mitteleuropa. Ergebnisse einer multizentrischen Studie der Arbeitsgemeinschaft “Resistenz” in der Paul-Ehrlich-Gesellschaft für Chemotherapie e.V. aus dem Jahre 1998. Chemother-J 9:51–86
Schouten MA, Hoogkamp-Konstantje JAA, Meis JFG, Voss A, and the European VRE Study Group (2000) Prevalence of vancomycin-resistant enterococci in Europe. Eur J Clin Microbiol Infect Dis 19:816–822
Goosens H, Jabes D, Rossi R, Lammens C, Privitera G, Courvalin P (2003) European survey of vancomycin-resistant enterococci in at-risk hospital wards and in vitro susceptibility testing of ramoplanin against these isolates. J Antimicrob Chemother 51(Suppl 3):5–12
Kirst HA, Thompson DG, Nicas TI (1998) Historical yearly usage of vancomycin. Antimicrob Agents Chemother 42:1303–1304
Jones RN (2001) Resistance patterns among nosocomial pathogens: trends over the past few years. Chest 119(Suppl 2):397S–404S
Talon D, Bertrand X, Thouverez M (2001) Risk factors and prevention of the acquisition and transmission of glycopeptide-resistant enterococci. Pathol Biol (Paris) 49:641–648
Harbarth S, Cosgrove S, Carmeli Y (2002) Effects of antibiotics on nosocomial epidemiology of vancomycin-resistant enterococci. Antimicrob Agents Chemother 46:1619–1628
Kolar M, Vagnerova I, Latal T, Urbanek K, Typovska H, Hubacek J, Papajik T, Raida L, Faber E (2002) The occurrence of vancomycin-resistant enterococci in hematological patients in relation to antibiotic use. New Microbiol 25:205–212
Donskey CJ, Helfand MS, Pultz NJ, Rice LB (2004) Effect of parenteral fluoroquinolone administration on persistence of vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. Antimicrob Agents Chemother 48:326–328
Rice LB, Hutton-Thomas R, Lakticova V, Helfand MS, Donskey CJ (2004) Beta-lactam antibiotics and gastrointestinal colonization with vancomycin-resistant enterococci. J Infect Dis 189:1113–1118
Willems RJ, Homan W, Top J, van Santen-Verheuvel M, Tribe D, Manzioros X, Gaillard C, Vanderbroucke-Grauls CM, Mascini EM, van Kregten E, van Embden JD, Bonten MJ (2001) Variant esp gene as a marker of a distinct genetic lineage of vancomycin-resistant Enterococcus faecium spreading in hospitals. Lancet 357:853–855
Homan W, Tribe D, Poznanski S, Li M, Hogg G, Spalburg E, van Embden JAD, Willems JL (2002) Multilocus sequence typing scheme for Enterococcus faecium. J Clin Microbiol 40:1963–1971
Top J, Schouls LM, Bonten MJ, Willems RJ (2004) Multiple-locus variable-number tandem repeat analysis, a novel typing scheme to study the genetic relatedness and epidemiology of Enterococcus faecium isolates. J Clin Microbiol 42:4503–4511
Willems RJ, Top J, van Santen M, Robinson DA, Coque TM, Baquero F, Grundmann H, Bonten MJ (2005) Global spread of vancomycin-resistant Enterococcus faecium from distinct nosocomial genetic complex. Emerg Infect Dis 11:821–828
Leavis H, Top J, Shankar N, Borgen K, Bonten M, van Embden J, Willems RJ (2004) A novel putative enterococcal pathogenicity island linked to the esp virulence gene of Enterococcus faecium and associated with epidemicity. J Bacteriol 186:672–682
Shankar V, Baghdayan AS, Huycke MM, Lindahl G, Gilmore MS (1999) Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect Immun 67:193–200
Coque TM, Willems R, Canton R, Del Campo R, Baquero F (2002) High occurrence of esp among ampicillin-resistant and vancomycin-susceptible Enterococcus faecium clones from hospitalized patients. J Antimicrob Chemother 50:1035–1038
Eaton TJ, Gasson MJ (2002) A variant enterococcal surface protein Espfm in Enterococcus faecium: distribution among food, commensal, medical, and environmental isolates. FEMS Microbiol Lett 216:269–275
Lund B, Edlund C (2003) Bloodstream isolates of Enterococcus faecium enriched with the enterococcal surface protein gene, esp, show increased adhesion to eukaryotic cells. J Clin Microbiol 41:5183–5185
Creti R, Imperi M, Bertuccini L, Fabretti F, Orefici G, Di Rosa R, Baldassarri L (2004) Survey for virulence determinants among Enterococcus faecalis isolated from different sources. J Med Microbiol 53:13–20
Shankar N, Lockatell CV, Baghdayan AS, Drachenberg C, Gilmore MS, Johnson DE (2001) Role of Enterococcus faecalis surface protein Esp in the pathogenesis of ascending urinary tract infection. Infect Immun 69:4366–4372
Toledo-Arana A, Valle J, Solano C, Arrizubieta MJ, Cucarella C, Lamata M, Amorena B, Leiva J, Penades JR, Lasa I (2001) The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl Environ Microbiol 67:4538–4545
Rice LB, Carias L, Rudin S, Vael C, Gossens H, Konstabel C, Klare I, Nallapareddy SR, Huang W, Murray BE (2003) A potential virulence gene, hyl Efm, predominates in Enterococcus faecium of clinical origin. J Infect Dis 187:508–512
Bonadio M, Meini M, Tagliaferri E, Gigli C, Vigna A (2000) Enterococcal glycopeptide resistance at an Italian teaching hospital. J Antimicrob Chemother 46:129–131
Henwood CJ, Livermore DM, Johnson AP, James D, Warner M, Gardiner A (2000) Susceptibility of gram-positive cocci from 25 UK hospitals to antimicrobial agents including linezolid. J Antimicrob Chemother 46:931–940
Reynold R, Potz N, Colman M, Williams A, Livermore D, MacGowan A, on behalf the BSAC Extended Working Party on Bacteria Resistance Surveillance (2004) Antimicrobial susceptibility of the pathogens of bacteraemia in the UK and Ireland 2001–2002: the BSAC Bacteraemia Resistance Surveillance Programme. J Antimicrob Chemother 53:1018–1032
European Antimicrobial Resistance Surveillance System (2004) Annual report 2003. http://www.earss.rivm.nl/PAGINA/DOC/EARSS%20annual%20report.2003.pdf, pp 36–38
Fahr A-M, Klare I, Eigner U, Turnwald-Maschler A, Holfelder M, Porsch G, Witte W (2004) Increasing incidence of vancomycin-resistant Enterococcus faecium isolates in German hospitals. 56. Jahrestagung der DGHM, Muenster, 26–29 September 2004, Poster IKP 002
Devriese LA, Pot B, Collins MD (1993) Phenotypic identification of the genus Enterococcus and differentiation of phylogenetically distinct enterococcal species and species groups. J Appl Bacteriol 75:399–408
Deutsches Institut für Normung (2004) DIN 58940. Medical microbiology—susceptibility testing of pathogens to antimicrobial agents. Part 8: microdilution. General method specific requirements. In: DIN Deutsches Institut für Normung eV (ed) DIN-Taschenbuch 222: Medizinische Mikrobiologie und Immunologie—Diagnostische Verfahren. Beuth-Verlag, Berlin, pp 342–353
Klare I, Konstabel C, Bastrop R (1997) Simple and rapid extraction of enterococcal DNA suitable for PCR of vancomycin resistance genes by use of the ion exchanger Chelex 100 resin. In: Methodischen Entwicklungen in der mikrobiologischen Nukleinsäure-Diagnostik, 3./4. Poster-Workshop, Dezember 1995/Dezember 1996, Berlin. Hyg Mikrobiol (Abstract-Band), Elsevier, Amsterdam, pp 33–34
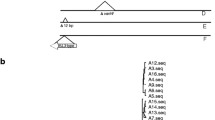
Tenover FC, Arbeit RD, Goering RV, Mickelsen PA, Murray BE, Persing DH, Swaminathan B (1995) Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol 33:2233–2239
Morrison D, Woodford N, Barrett SP, Sisson P, Cookson BD (1999) DNA banding pattern polymorphism in vancomycin-resistant Enterococcus faecium and criteria of strain definition. J Clin Microbiol 37:1084–1091
Claus H, Cuny C, Pasemann B, Witte W (1996) A database system for fragment patterns of genomic DNA of Staphylococcus aureus. Zentralbl Bateriol 287:105–116
Woodford N, Tysall L Auckland C, Stockdale MW, Lawson AJ, Walker RA, Livermore DM (2002) Detection of oxazolidinone-resistant Enterococcus faecalis and Enterococcus faecium strains by real-time PCR and PCR-restriction fragment length polymorphism analysis. J Clin Microbiol 40:4298–4300
Werner G, Strommenger B, Klare I, Witte W (2004) Molecular detection of linezolid resistance in Enterococcus faecium and Enterococcus faecalis by use of 5′ nuclease real-time PCR compared to a modified classical approach. J Clin Microbiol 42:5327–5331
Stampone L, Del Grosso M, Boccia D, Pantosi A (2005) Clonal spread of a vancomycin-resistant Enterococcus faecium strain among bloodstream-infecting isolates in Italy. J Clin Microbiol 43:1575–1580
Klare I, Konstabel C, Badstubner D, Werner G, Witte W (2003) Occurrence and spread of antibiotic resistances in Enterococcus faecium. Int J Food Microbiol 88:269–290
Suppola JP, Kolho E, Salmenlinna S, Tarrka E, Vuopio-Varkila J, Vaara M (1999) vanA and vanB incorporate into an endemic ampicillin-resistant vancomycin-sensitive Enterococcus faecium strain: effect on interpretation of clonality. J Clin Microbiol 37:3934–3939
Kawalec M, Gniadkowski M, Hryniewicz W (2000) Outbreak of vancomycin-resistant enterococci in a hospital in Gdansk, Poland, due to horizontal transfer of different Tn1546-like transposon variants and clonal spread of several strains. J Clin Microbiol 38:3317–3322
Kawalec M, Gniadkowski M, Zaleska M, Ozorowski T, Konopka L, Hryniewicz W (2001) Outbreak of vancomycin-resistant Enterococcus faecium of the phenotype VanB in a hospital in Warsaw, Poland: probable transmission of the resistance determinants into an endemic vancomycin-susceptible strain. J Clin Microbiol 39:1781–1787
Woodford N, Chadwick PR, Morrison D, Cookson BD (1997) Strains of glycopeptide-resistant Enterococcus faecium can alter their van genotypes during an outbreak. J Clin Microbiol 35:2966–2968
Oanca C, Klare I, Witte W, Werner G (2004) Conjugative transfer of the virulence gene, esp, among isolates of Enterococcus faecium and Enterococcus faecalis. J Antimicrob Chemother 54:232–235
Bonora MG, Ligozzi M, De Fatima M, Bragagnolo L, Goglio A, Guazzotti GC, Fontana R (2004) Vancomycin-resistant Enterococcus faecium isolates causing hospital outbreaks in northern Italy belong to the multilocus sequence typing C1 lineage. Microb Drug Resist 10:114–123
Coque TM, Willems RJ, Fortun J, Top j, Diz S, Loza E, Canton R, Baquero F (2005) Population structure of Enterococcus faecium causing bacteremia in a Spanish university hospital: setting the scene for a future increase in vancomycin resistance? Antimicrob Agents Chemother 49:2693–2700
Donskey CJ (2004) The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin Infect Dis 39:219–226
Torres-Viera C, Dembry LM (2004) Approaches to vancomycin-resistant enterococci. Curr Opin Infect Dis 17:541–547
Brauers UJ, Kresken M, Hafner D, Shah PM, and The German Linezoid Resistance Study Group (2005) Surveillance of linezolid resistance in Germany, 2001–2002. Clin Microbiol Infect 11:39–46
Johnson AP, Tysall L, Stockdale MV, Woodford N, Kaufmann ME, Warner M, Livermore DM, Asboth F, Allerberger FJ (2002) Emerging linezolid-resistant Enterococcus faecalis and Enterococcus faecium isolated from two Austrian patients in the same intensive care unit. Eur J Clin Microbiol Infect Dis 21:751–754
Auckland C, Teare L, Cooke F, Kaufmann ME, Warner M, Jones G, Bamford K, Ayles H, Johnson AP (2002) Linezolid-resistant enterococci: report of the first isolates in the United Kingdom. J Antimicrob Chemother 50:743–746
Rahim S, Pillai SK, Gold HS, Venkataraman L, Inglima K, Press RA (2003) Linezolid-resistant, vancomycin-resistant Enterococcus faecium infection in patients without prior exposure to linezolid. Clin Infect Dis 36:e146–e148
Krawczyk B, Samet A, Bronk M, Hellmann A, Kur J (2004) Emerging linezolid-resistant, vancomycin-resistant Enterococcus faecium from a patient of a haematological unit in Poland. Pol J Microbiol 53:193–196
Halle E, Padberg J, Rosseau S, Klare I, Werner G, Witte W (2004) Linezolid-resistant Enterococcus faecium and Enterococcus faecalis isolated from a septic patient: report of first isolates in Germany. Infection 32:182–183
Brooks S, Khan A, Stoica D, Griffith J, Friedeman L, Mukherji R, Hameed R, Schupf N (1998) Reduction in vancomycin-resistant Enterococcus and Clostridium difficile infections following change to tympanic thermometers. Infect Control Hosp Epidemiol 19:333–336
Hospital Infection Control Practices Advisory Committee (1995) Recommendations for preventing the spread of vancomycin resistance. Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep 44(RR-12):1–13
Muto CA, Jernigan JA, Ostrowsky BE, Richet HM, Jarvis WR, Boyce JM, Farr BM, for the Society for Healthcare Epidemiology of America (2003) SHEA guidelines for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect Control Hosp Epidemiol 24:286–362
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klare, I., Konstabel, C., Mueller-Bertling, S. et al. Spread of ampicillin/vancomycin-resistant Enterococcus faecium of the epidemic-virulent clonal complex-17 carrying the genes esp and hyl in German hospitals. Eur J Clin Microbiol Infect Dis 24, 815–825 (2005). https://doi.org/10.1007/s10096-005-0056-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-005-0056-0