Summary
Background
Combination of a cell wall-active antibiotic with an aminoglycoside confers a synergistic effect in the treatment of some severe enterococcal infections. Unfortunately, with the emergence of enterococci with high-level resistance to aminoglycosides, particularly to gentamicin, the efficacy of the synergistic combinations has decreased. In this study, high-level gentamicin-resistant (HLGR) isolates of enterococci and the diversity of the genes encoding aminoglycoside-modifying enzymes (AMEs) as well as putative clonal dissemination of HLGR isolates were investigated in a university hospital in southeastern Iran.
Methods
The minimum inhibitory concentration of gentamicin was determined and HLGR isolates were investigated for AME genes. Genetic similarity between isolates was analyzed using repetitive extragenic palindromic (rep)-Polymerase Chain Reaction (PCR) assay.
Results
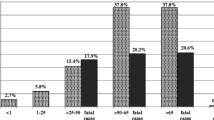
Of 150 Enterococcus isolates, 62 isolates including Enterococcus faecalis (n = 46) and E. faecium (n = 16) were identified as HLGR. The most prevalent AME genes in both species were as follows: aph(3′)-IIIa (n = 44), aac(6′)-Ie-aph(2′)-Ia (n = 36), and ant(4′)-Ia (n = 15). The rep-PCR analysis showed clonality among E. faecalis isolates, so that 27 isolates were grouped in seven clusters representing similarity greater than 95%.
Conclusions
No link between AME determinants and clusters was found. Clonal spread of HLGR isolates of E. faecalis was found within our hospital. More rigorous recommendations are required to avoid dissemination of such resistant microorganisms in the hospital setting.
Zusammenfassung
Grundlagen
Die Kombination eines zellwandaktiven Antibiotikums mit einem Aminoglykosid bewirkt einen synergistischen Effekt bei der Behandlung einiger schwerer Enterokokkeninfektionen. Leider hat durch das Auftreten von Enterokokken mit einer hohen Resistenz gegen Aminoglykoside, insbesondere gegen Gentamicin, die Wirksamkeit der synergistischen Kombinationen abgenommen. In dieser Studie wurden gentamicinresistente (HLGR) Isolate von Enterokokken und die Vielfalt der Gene, die für aminoglycosidmodifizierende Enzyme (AME) kodieren, sowie die mutmaßliche klonale Verbreitung von HLGR-Isolaten in einem Universitätskrankenhaus im Südosten des Iran untersucht.
Methodik
Die minimale Hemmkonzentration von Gentamicin wurde bestimmt, und die HLGR-Isolate wurden für AME-Gene untersucht. Die genetische Ähnlichkeit zwischen den Isolaten wurde mit einem repetitiven palindromischen (rep‑)PCR(Polymerasekettenreaktion)-Assay analysiert.
Ergebnisse
Von 150 Enterococcus-Isolaten wurden 62 Isolate, darunter Enterococcus faecalis (n = 46) und E. faecium (n = 16), als HLGR identifiziert. Die häufigsten AME-Gene in beiden Spezies waren wie folgt: aph(3′)-IIIa (n = 44), aac(6′)-Ie-aph(2′)-Ia (n = 36) und ant(4′)-Ia (n = 15). Die rep-PCR-Analyse zeigte eine Klonalität unter den E.-faecalis-Isolaten, sodass 27 Isolate mit einer Ähnlichkeit von mehr als 95 % in gesamt 7 Clustern gruppiert werden konnten.
Schlussfolgerungen
Es wurde kein Zusammenhang zwischen AME-Determinanten und Clustern gefunden. In dem Krankenhaus der Autoren wurde eine klonale Ausbreitung von HLGR-Isolaten von E. faecalis festgestellt. Es sind strengere Empfehlungen erforderlich, um die Verbreitung solcher resistenter Mikroorganismen im Krankenhaus zu vermeiden.

Similar content being viewed by others
Abbreviations
- AACs/APHs:
-
acetyltransferases/phosphotransferases
- AMEs:
-
aminoglycoside-modifying enzymes
- ANTs:
-
adenyltransferases
- APHs:
-
phosphotransferases
- CLSI:
-
Clinical and Laboratory Standard Institute
- HLGR:
-
high-level gentamicin-resistant
- MIC:
-
minimum inhibitory concentration
- rep-PCR:
-
repetitive extragenic palindromic
References
Hollenbeck BL, Rice LB. Intrinsic and acquired resistance mechanisms in enterococcus. Virulence. 2012;3:421–569. https://doi.org/10.4161/viru.21282.
Arias CA, Contreras GA, Murray BE. Management of multidrug-resistant enterococcal infections. Clin Microbiol Infect. 2010;16:555–62. https://doi.org/10.1111/j.1469-0691.2010.03214.x.
Rosvoll TC, Lindstad BL, Lunde TM, et al. Increased high-level gentamicin resistance in invasive enterococcus faecium is associated with aac (6′) ie-aph (2 ″) Ia-encoding transferable megaplasmids hosted by major hospital-adapted lineages. FEMS Immunol Med Microbiol. 2012;66:166–76. https://doi.org/10.1111/j.1574-695X.2012.00997.x.
Behnood A, Farajnia S, Moaddab SR, et al. Prevalence of aac (6′)-Ie-aph (2 ″)-Ia resistance gene and its linkage to Tn5281 in enterococcus faecalis and enterococcus faecium isolates from Tabriz hospitals. Iran J Microbiol. 2013;5:203–8.
Emaneini M, Khoramian B, Jabalameli F, et al. Prevalence of high-level gentamicin-resistant enterococcus faecalis and enterococcus faecium in an Iranian hospital. J Prev Med Hyg. 2016;57:197–200.
Kuch A, Willems RJ, Werner G, et al. Insight into antimicrobial susceptibility and population structure of contemporary human enterococcus faecalis isolates from Europe. J Antimicrob Chemother. 2011;67:551–8. https://doi.org/10.1093/jac/dkr544.
Singh N, Léger MM, Campbell J, Short B, Campos JM. Control of vancomycin-resistant enterococci in the neonatal intensive care unit. Infect Control Hosp Epidemiol. 2005;26:646–9. https://doi.org/10.1086/502595.
Akpaka PE, Kissoon S, Jayaratne P. Molecular analysis of vancomycin-resistant enterococci isolated from regional hospitals in Trinidad and Tobago. Adv Med. 2016; https://doi.org/10.1155/2016/8762691.
Facklam RR, Collins MD. Identification of enterococcus species isolated from human infections by a conventional test scheme. J Clin Microbiol. 1989;27:731–4.
Dutka-Malen S, Evers S, Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol. 1995;33:24–7.
Clinical and Labratory Standard Institute (CLSI). Performance standards for antimicrobial susceptibility testing. 28th ed. Wayne, PA: CLSI; 2015. pp. M100–S23.
Vakulenko SB, Donabedian SM, Voskresenskiy AM, et al. Multiplex PCR for detection of aminoglycoside resistance genes in enterococci. Antimicrob Agents Chemother. 2003;47:1423–6.
Namdari H, DelVecchio V. Application of PCR for the characterization of enterococci. Clin Microbiol Newsl. 1998;20:91–4.
Sreeja S, Sreenivasa Babu PR, Prathab AG. The prevalence and the characterization of the enterococcus species from various clinical samples in a tertiary care hospital. J Clin Diagn Res. 2012;6:1486–8. https://doi.org/10.7860/JCDR/2012/4560.2539.
Dadfarma N, Fooladi AA, Oskoui M, et al. High level of gentamicin resistance (HLGR) among enterococcus strains isolated from clinical specimens. J Infect Public Health. 2013;6:202–8. https://doi.org/10.1016/j.jiph.2013.01.001.
Padmasini E, Padmaraj R, Ramesh SS. High level aminoglycoside resistance and distribution of aminoglycoside resistant genes among clinical isolates of enterococcus species in Chennai, India. ScientificWorldJournal. 2014;2014:1–5. https://doi.org/10.1155/2014/329157.
Amini F, Krimpour HA, Ghaderi M, et al. Prevalence of aminoglycoside resistance genes in enterococcus strains in Kermanshah, Iran. Iran J Med Sci. 2018;43:487–93.
Hasani A, Sharifi Y, Ghotaslou R, et al. Molecular screening of virulence genes in high-level gentamicin-resistant enterococcus faecalis and enterococcus faecium isolated from clinical specimens in northwest Iran. Indian J Med Microbiol. 2012;30:175–81. https://doi.org/10.4103/0255-0857.96687.
Li W, Li J, Wei Q, et al. Characterization of aminoglycoside resistance and virulence genes among enterococcus spp. isolated from a hospital in China. Int J Environ Res Public Health. 2015;12:3014–25. https://doi.org/10.3390/ijerph120303014.
Tomita H, Pierson C, Lim SK, et al. Possible connection between a widely disseminated conjugative gentamicin resistance (pMG1-like) plasmid and the emergence of vancomycin resistance in enterococcus faecium. J Clin Microbiol. 2002;40:3326–33. https://doi.org/10.1128/JCM.40.9.3326-3333.2002.
Woegerbauer M, Zeinzinger J, Springer B, et al. Prevalence of the aminoglycoside phosphotransferase genes aph (3′)-IIIa and aph (3′)-IIa in escherichia coli, enterococcus faecalis, enterococcus faecium, pseudomonas aeruginosa, salmonella enterica subsp. enterica and staphylococcus aureus isolates in Austria. J Med Microbiol. 2014;63:210–7. https://doi.org/10.1099/jmm.0.065789-0.
Khani M, Fatollahzade M, Pajavand H, et al. Increasing prevalence of aminoglycoside-resistant enterococcus faecalis isolates due to the aac (6′)-aph (2″) gene: a therapeutic problem in Kermanshah, Iran. Jundishapur J Microbiol. 2016;9:e28923. https://doi.org/10.5812/jjm.28923.
Niu H, Yu H, Hu T, et al. The prevalence of aminoglycoside-modifying enzyme and virulence genes among enterococci with high-level aminoglycoside resistance in Inner Mongolia, China. J Microbiol. 2016;47:691–6. https://doi.org/10.1016/j.bjm.2016.04.003.
Funding
This work was supported by the Student Research Committee of Kerman University of Medical Sciences, Kerman, Iran (Grant No: 96000503).
Author information
Authors and Affiliations
Contributions
F. Saffari conceived the study and participated in its design and execution, the data analysis, and writing the manuscript. H. Darehkordi contributed to sample collection and processing. R. Ahmadrajabi participated in design and execution of the study and writing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
F. Saffari, H. Darehkordi, and R. Ahmadrajabi declare that they have no competing interests.
Ethical standards
All procedures performed in studies involving human participants or on human tissue were in accordance with the ethical standards of the institutional and/or national research committee (ethics committee of Kerman University of Medical Sciences (IR.KMU.REC.1396.1599)) and with the 1975 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Saffari, F., Darehkordi, H. & Ahmadrajabi, R. Clonal dissemination of high-level gentamicin-resistant isolates of Enterococcus faecalis within a university hospital in southeastern Iran. Wien Med Wochenschr 171, 18–23 (2021). https://doi.org/10.1007/s10354-019-00716-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10354-019-00716-2
Keywords
- Minimum inhibitory concentration
- Aminoglycoside modifying enzyme
- Enterococcus faecalis
- High-level gentamicin-resistant
- Repetitive extragenic palindromic (rep)-PCR