Abstract
Objective
To exp lore changes in immunoglobulin (Ig) levels for people with relapsing-multiple sclerosis (RMS) treated with ocrelizumab or ofatumumab and the relationship between Ig levels and infections.
Methods
A systematic literature review (SLR) was conducted to identify clinical trials and real-world evidence (RWE) studies on Ig levels over time and studies on associations with infections for ocrelizumab and ofatumumab for people with RMS through 10 September 2021. Searches were conducted in Embase, MEDLINE, Cochrane Library, trial registries, and recent conference abstracts.
Results
Of 1,580 articles identified, 30 reporting on 11 trials and 5 RWE studies were included. Ocrelizumab trials (n = 4) had 24–336 weeks of follow-up and reported decreasing Ig G (IgG) levels, while RWE (n = 5) had 52–78 weeks of follow-up and reported IgG to be stable or decrease only slightly. IgG levels were stable in ofatumumab trials (n = 5; 104–168 weeks of follow-up), but no RWE or longer-term studies were identified. No apparent association between decreased Ig levels and infections was observed during ofatumumab treatment (ASCLEPIOS I/II), while for ocrelizumab, the only data on apparent associations between decreased IgG levels and serious infection rates were for a pooled population of people with RMS or primary progressive MS.
Conclusion
Decreasing IgG levels have been correlated with increased infection risk over time. IgG levels appeared to decrease over time in ocrelizumab trials but remained relatively stable over time in ofatumumab trials. Additional research is needed to understand differences between ocrelizumab and ofatumumab and identify people at risk of decreasing IgG levels and infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Multiple sclerosis (MS) is a chronic autoimmune disease characterized by transient alterations in the blood–brain barrier, inflammation, demyelination, and neurodegeneration [1]. Relapsing MS (RMS) is the most common subtype of MS, typically beginning with a relapsing and remitting course that, after several years, in a subset of people, may transition to a clinical phenotype that is instead characterized by gradual neurological decline. This is referred to as secondary progressive MS (SPMS). On the other hand, approximately 10% of people with MS (PwMS) experience progressive accumulation of neurologic disability from the outset, termed primary progressive MS (PPMS) [1, 2]. Although it is common to differentiate MS subtypes according to these clinical phenotypes, rather than being clearly differentiated, these subtypes may instead form a continuum, representing different phases or stages of the same MS disease process as it evolves. Earlier in the MS disease course, adaptive over innate immune system–mediated inflammation is thought to predominate. Although innate immune system dysfunction may increase over time and potentially predominate in progressive MS, in later disease, adaptive immune dysfunction remains relevant [3, 4]. For example, meningeal B cell follicles are more common in progressive MS than relapsing–remitting multiple sclerosis (RRMS), and the same antigen-experienced B cell clones have been shown to present in the brain parenchyma, as in the meningeal follicles of PwMS [3, 5].
In people with RMS, treatment with the anti-CD20 monoclonal antibodies ofatumumab and ocrelizumab, both approved by the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), has been shown to delay disease progression, reduce relapses, and reduce new gadolinium enhancing and/or T2 lesion formation on magnetic resonance imaging brain scans [1, 6]. While the precise mechanism by which ofatumumab and ocrelizumab exert their therapeutic effects in MS is unknown, both are cytolytic monoclonal antibodies presumed to involve binding to CD20, a cell surface antigen present on pre-B and mature B lymphocytes. Following cell surface binding to B lymphocytes, ofatumumab and ocrelizumab result in antibody-dependent cellular cytolysis and complement-mediated lysis. The resulting immunosuppression may lead to an increased risk of serious infections. For example, among PwMS in the Swedish MS registry, treatment with the related anti-CD20 monoclonal antibody rituximab was associated with approximately 3 times greater odds of hospitalization for infection with COVID-19 relative to other disease-modifying therapies combined [7]. Ofatumumab may have a number of theoretical advantages with regard to immunosuppression, including administration via subcutaneous injection versus intravenous infusion and at a lower dose than ocrelizumab, resulting in a greater lymphatic compartment effect and overall less potential for deeper B cell depletion, as well as faster B cell repletion after discontinuation, and limited recovery of circulating B cells [8,9,10].
Some evidence from clinical trials and observational studies has suggested an association between Ig antibody levels and infection rates, as well as infection severity in PwMS. In particular, an association between reduced serum immunoglobulin G (IgG) and increased infection risk has been suggested [11]. Having an improved understanding of such risks is particularly relevant within the context of B cell–depleting therapeutic strategies because one of the main functions of B cells is antibody production. In a broader sense, people with RMS taking disease-modifying therapies (DMTs) that may interfere with the generation and/or release of Igs in response to infectious exposures may accordingly have a greater risk for serious infections—a particularly important therapeutic consideration during the COVID-19 pandemic. On the contrary, and somewhat unsurprisingly, because IgA plays an important role in adaptive immune protection at mucosal surfaces, it has been suggested that higher levels of serum IgA may be related to decreased infection risk [12]. Multiple clinical trials and real-world studies have reported Ig levels over time for patients with MS treated with ofatumumab and ocrelizumab. For example, the phase 3 ASCLEPIOS I/II study, with safety data up to 4 years, showed that mean IgG levels remained similar to baseline values for patients who used ofatumumab, and no associated increased risk of serious infections was reported [13].
In this systematic literature review (SLR), our principal objective was to review published data from randomized controlled trials (RCTs) and real-world evidence (RWE) studies on Ig levels over time between people with RMS treated with the currently approved anti-CD20 monoclonal antibodies, ofatumumab and ocrelizumab. Moreover, we also sought to determine if the incidence and severity of infectious disease adverse events correlate with Ig levels in people with RMS being treated with either ocrelizumab or ofatumumab.
Methods
The target population for this SLR was people with RMS (including those with RRMS or with active SPMS) who were treated with either ocrelizumab or ofatumumab in either a clinical trial or an observational study setting.
The outcomes of interest from the included studies, where available, were as follows: mean or median IgM, IgG, and IgA levels at baseline and follow-up timepoints while on treatment with ocrelizumab or ofatumumab and their comparator DMTs, if applicable; changes in mean or median IgM, IgG, and IgA from baseline to different timepoints; and the percentage of the study population below a given threshold for IgM, IgG, and IgA at baseline and at different timepoints while on treatment.
Methods of this SLR were consistent with those outlined in the Cochrane Handbook for Systematic Reviews of Interventions [14]. Following a study protocol with prespecified search terms, experienced research librarians conducted electronic searches to identify English-language publications with publication dates from the initiation of the databases searched until 10 September 2021. We placed no limitation on geography and searched the following databases: MEDLINE and MEDLINE In-Process (using the PubMed platform); Embase (using the Elsevier Platform); and the Cochrane Library, including the Cochrane Central Register of Controlled Trials (CENTRAL) and the Cochrane Database of Systematic Reviews. In addition, selected conference proceedings, trial registries, and regulatory websites were searched. To minimize the risk of missing eligible studies, we also performed a manual search by screening the bibliographies of identified SLRs and meta-analyses and included studies.
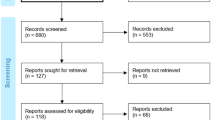
Screenings of publications for inclusion were based on prespecified inclusion and exclusion criteria (Table 1). Publications were screened by 1 researcher with a 10% random check conducted by a second researcher. The inclusion and exclusion process was documented using a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram (see Fig. 1).
PRISMA diagram for the systematic literature review. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses [15]. aThe category “Other” includes duplicate references and conference abstracts before 2017
One reviewer abstracted data from included studies into detailed evidence tables (see Appendix A, Supplemental Material); a second reviewer checked all abstractions against the original source. Data in the evidence tables included information on study authors, year, country, and funding source; RMS population(s) studied; trial design or data source used; and other study characteristics. The evidence tables also included key study endpoints. Where key information was available within figures only, we digitized these data using DigitizeIt software (DigitizeIt; I. Bormann; Braunschweig, Germany), and the digitized data were checked by a second researcher. Owing to heterogeneity in the studies and reporting of outcomes, and the lack of common comparators across the studies, indirect comparison of aggregate data was not considered to be feasible. Therefore, no statistical analysis was conducted; here, we present a summary of the data reported in the identified studies separately.
Results
SLR results
The electronic database, internet searches, hand searches, and registry searches were conducted 10–16 September 2021 and yielded a total of 1,580 records (titles and abstracts) for manual level 1 screening of titles and abstracts (databases = 1,316; internet searches = 124; hand searches = 13; registry searches = 127). After the initial (level 1) screening of titles and abstracts according to the inclusion and exclusion criteria in Table 1, 712 publications (database searches = 492; internet searches = 106; hand searches = 6; registry searches = 108) were progressed to level 2 full-text screening. At the level 2 screening, 30 publications (database searches = 19; internet searches = 10; hand searches = 0; registry searches = 1) met the predefined inclusion criteria and thus were selected for data extraction. Figure 1 depicts the volume of publications included and excluded at each stage of screening in a PRISMA flow diagram.
Study characteristics
Clinical trials
The 24 included trial publications reported data on 11 trials (ASCLEPIOS I and II, APLIOS, APOLITOS, ALITHIOS, OBOE, OPERA I and II, OMS115102, VELOCE, and NCT00676715) (Table 2). Of these, OPERA I and II were reported only as pooled data. APLIOS, APOLITOS, and ALITHIOS were reported only as pooled data with ASCLEPIOS I and II, whereas ASCLEPIOS I and II each were reported separately and in addition were pooled. Therefore, results from 9 trial populations were included.
Of the 11 included trials, ASCLEPIOS I and II, APLIOS, ALITHIOS, OBOE, OPERA I and II, OMS115102, and NCT00676715 were multinational; APOLITOS was conducted in Japan and Russia; and VELOCE was conducted in the United States (US) and Canada. Ocrelizumab was studied in 5 trials (OBOE, OPERA I and II, VELOCE, and NCT00676715), and ofatumumab in 6 trials (OMS115102, ASCLEPIOS I and II, APLIOS, APOLITOS, and ALITHIOS). ASCLEPIOS I and II [16] were phase 3, multicenter, double-blind, RCTs sponsored by Novartis Pharmaceuticals. The trials included people with RMS who received treatment with either ofatumumab (n = 946) or teriflunomide (n = 936) for up to 130 weeks. Wiendl et al. [17] further pooled ASCLEPIOS I and II data with the phase 2 RCT APOLITOS, phase 2 RCT APLIOS, and the ongoing, phase 3, open-label, 5-year extension ALITHIOS trial (with 3.5 years of data available at the time of this SLR), all of which included people with RMS treated with ofatumumab throughout and were sponsored by Novartis Pharmaceuticals. VELOCE [19] and OPERA I and II [20] were also phase 3 RCTs, both of which were sponsored by Hoffmann-La Roche. VELOCE [19] was a 24-week, phase 3, open-label, multicenter RCT that included assessment of the effect of ocrelizumab treatment on response to vaccines. This trial included adults with RRMS who were randomized either to ocrelizumab (n = 68) or to a control group (n = 34) in which people either continued their current interferon beta therapy or received no DMT. OPERA I and II [20] were phase 3 multicenter, double-blind RCTs that included people with RMS who were randomly assigned to receive ocrelizumab at a dose of 600 mg by means of intravenous infusion every 24 weeks (n = 410, OPERA I; n = 417, OPERA II) or interferon beta-1a (n = 411, OPERA I; n = 418, OPERA II) at a dose of 44 μg administered subcutaneously 3 times weekly throughout the 96-week treatment period.
OMS115102 [21] was a phase 2 multicenter, double-blind, crossover, dose-finding RCT that was sponsored by GlaxoSmithKline. This trial included people with RRMS who received treatment with either ofatumumab or placebo across 6 experimental cohorts receiving varying doses of intravenous ofatumumab (100 mg, 300 mg, or 700 mg; n = 26) crossing over to placebo, or placebo crossing over to intravenous ofatumumab (n = 12) during a 48-week treatment period. NCT00676715 [22] was also a phase 2 RCT with a parallel-group, double-blind design sponsored by Genentech, Inc. This trial included people with RRMS who were randomized to either 2 placebo intravenous infusions at 15-day intervals (n = 54), 2 infusions of 300-mg ocrelizumab at 15-day intervals with infusion reaction prophylaxis (n = 55), or open-label 30-μg interferon (IFN) β administered intramuscularly once a week (n = 54) over a 72-week treatment period.
OBOE [18] was the only phase 4 open-label RCT trial identified in this SLR. This trial was also sponsored by Genentech, Inc., and included a population of 79 of 100 total people with RMS with available cerebrospinal fluid (CSF) samples who were treated with ocrelizumab and had undergone lumbar punctures. Ocrelizumab was administered as two 300-mg intravenous (IV) infusions on Days 1 and 15, then as a single infusion of 600 mg on weeks 24 and 48. Participants received a lumbar puncture before the start of dosing with ocrelizumab and a second lumbar puncture at week 12.
Among the 11 included trials, 5 trials required a diagnosis of RMS in accordance with the 2010 revised McDonald criteria, 5 trials specified a participant age range between 18 and 55 years, and all required an Expanded Disability Status Scale (EDSS) score, which ranged between 0 and 6 across trials. Detailed tables presenting the study design for each trial, including eligibility criteria (Table S1) and baseline characteristics (Table S2), are included in Appendix A (Supplemental Material).
Real-world studies
Six RWE publications reported data on 5 studies (Table 2), all of which studied ocrelizumab. Of the included studies, 2 were conducted in the US, 1 in the Netherlands, 1 in Italy, and 1 in Spain. Three studies were retrospective and 2 were prospective. The studies had small sample sizes ranging from 42 to 161 participants, with follow-up times ranging from 52 to 78 weeks; 3 of the 6 included publications were conference abstracts only. Detailed tables presenting the study design, including eligibility criteria (Table S3) and baseline characteristics (Table S4), for the RWE studies are included in Appendix A (Supplemental Material).
Key outcomes
Among the clinical trial studies included in the review, for both IgG and IgM levels, mean or median values were reported in 7 of the 9 included trial populations, change from baseline in 6, and percentage of participants achieving a certain level in 3 trial populations (Table 2). IgA data were reported the least across the trials, with mean or median values for IgA reported in just 1 trial population, change from baseline in 2 trial populations, and percentage of people achieving a certain level in just 1 trial population. Of the 9 trial populations included in the SLR, only 3 reported data on the association of Ig levels with infection. Most data came from four large phase 3 RCTs: ASCLEPIOS I and II [16, 29,30,31,32,33] and OPERA I and II [20, 34,35,36,37,38,39,40]. NCT00676715 reported limited data [22]. While the VELOCE trial did report change in Ig levels, the trial focused on immune responses and the effectiveness of vaccinations in ocrelizumab-treated people over only 24 weeks [19]. OBOE only reported cerebrospinal fluid (CSF) Ig levels [18], while OMS115102 investigated much higher intravenous doses of ofatumumab than the marketed subcutaneous dose and included only 38 participants [21].
Among the real-world studies included in the review, mean or median IgG levels were reported for all 5 of the included studies, and IgG change from baseline and percentage of individuals achieving a certain level were reported in 1 study each (Table 2). Mean or median IgM levels were reported in 4 of the 5 RWE studies, with IgM change from baseline values reported in just 1 study. No data were provided for IgA levels in any of the included RWE studies.
IgG
The most reported outcome was change in IgG levels (Fig. 2; Table S5, Appendix B). Four trial populations of ocrelizumab with 24 weeks to 336 weeks of follow-up reported a decrease in IgG levels over time. In 5 trial populations of ofatumumab with 104 to 168 weeks of follow-up, a transient decrease in IgG levels occurred at week 48, but decreases in IgG levels were not observed at later time points (Fig. 2).
Mean/median percentage change from baseline in IgG levels: clinical trials of ocrelizumab and ofatumumab. CFB = change from baseline; CSF = cerebrospinal fluid; IgG = immunoglobulin G; LLN = lower limit of normal. Note: Changes in IgG levels are for summary purposes only; owing to heterogeneity in trial designs and outcomes, cross-trial comparisons should not be made. Detailed outcomes of clinical trials are presented in Appendix B, Supplemental Material. OBOE examined only CSF IgG levels and not serum IgG levels. ASCLEPIOS I/II: median IgG levels are presented; LLN for serum IgG defined as 5.65 g/L [17]. VELOCE: mean IgG levels are presented; LLN for serum IgG defined as 4.6 g/L [37]. OPERA I/II: mean IgG levels are presented; LLN for serum IgG defined as 5.65 g/L [35]. NCT00676715: mean IgG levels are presented. ASCLEPIOS I/II, APLIOS, APOLITOS, ALITHIOS pooled: mean IgG levels are presented; LLN for serum IgG defined as 7.0 g/L [29]. Treatment interruption due to notably low IgG levels (> 20% below LLN) and treatment discontinuation were reported for 0.1% and 0.2% of patients, respectively [17]
In the OPERA I and II trials, a decrease in mean IgG levels was observed for the ocrelizumab treatment arm over 336 weeks [38]. These trials reported a mean change of –5% at week 96 and of –17% at week 264 in the ocrelizumab arm (Fig. 2) [36, 38]. In the IFN β-1a arm, after a mean increase up to week 96, a decrease was subsequently observed after the switch to ocrelizumab treatment (Table S5) [38]. Over a period of up to 7 years, IgG levels decreased at an average rate of 0.33 g/L per year (− 3% per year). At the latest recorded timepoint of week 312, 7.7% of people treated with ocrelizumab throughout had an IgG level less than the lower level of normal (LLN). NCT00676715 also reported a mean reduction (–6.94% vs baseline) in IgG level at week 120 in people who received 4 cycles of ocrelizumab [22] (Fig. 2). In the VELOCE study, mean IgG levels were 10.25 g/L at baseline, 10.36 g/L at week 12, and 10.21 g/L at week 24, although it should be noted that this vaccine response study had a much shorter duration of only 24 weeks.
In the ASCLEPIOS I and II trials, a transient drop in median IgG levels was observed with ofatumumab, returning to baseline value by week 72. These trials reported a mean change of –4.3% at week 48 and of + 2.2% at week 96 [41] (Fig. 2). Mean IgG levels remained stable over up to 3.5 years of treatment and above the LLN of 5.65 g/L [17]. In the pooled ASCLEPIOS I/II, APOLITOS, APLIOS, and ALITHIOS population, after a transient drop through week 48 IgG levels returned to baseline levels, which were maintained across 3.5 years of ofatumumab treatment [17] (Fig. 2).
Real-world studies (all of which studied ocrelizumab) generally reported that IgG levels decreased slightly over time or remained stable, although results were variable and it was often unclear if any decrease was statistically significant (see Table S6, Appendix B). The RWE studies performed by Lopez Ruiz et al. [28], Evertsson et al. [26], and Edgar et al. [25] all reported similar patterns in which an overall decrease in median and mean IgG levels over time was observed with ocrelizumab treatment (Fig. 3 and Table S6). That being said, information on statistical significance was not reported by Edgar et al. [25] or Lopez Ruiz et al. [28]; in the study by Evertsson et al. [26], the change in IgG levels was significant in one analysis but not in another. More specifically, Evertsson et al. [26] reported a mean change of –0.16 g/L (95% confidence interval [CI], − 0.31 to − 0.01; P = 0.039) with each ocrelizumab infusion by mixed-effects modeling, but analysis by generalized estimating equations was not significant (P = 0.102). They further reported mean IgG levels by subgroups, with the largest decrease at 52 weeks found in people aged > 50 years. Lopez Ruiz et al. [28] reported that no participants exhibited IgG levels < LLN at 78 weeks. It should also be noted that Prezioso et al. [23] (in a single-arm interventional study) and van Lierop et al. [24] (in a cohort study of individuals switching from natalizumab to ocrelizumab either directly or indirectly because of progressive multifocal leukoencephalopathy risk) reported slight increases from baseline in mean and median IgG levels over time with ocrelizumab treatment. Prezioso et al. [23] reported that IgG levels had a stationary trend over time (P < 0.05) during the 12 months ocrelizumab treatment, whereas van Lierop et al. [24] did not comment on the results. However, both studies had sample sizes of only 42 participants (Fig. 3 and Table S6). Lopez Ruiz et al. [28] also reported an increase from baseline to week 52, before levels decreased.
Mean IgG levels over time with ocrelizumab treatment: real-world studies. IgG = immunoglobulin G. Note: IgG levels over time are for summary purposes only; owing to heterogeneity in study designs and outcomes, cross-study comparisons should not be made. Detailed outcomes of real-world studies are presented in Appendix B, Supplemental Material
IgM
IgM was reported to decrease over time in both ocrelizumab and ofatumumab trials (Fig. 4, Table S6). In the same trials that reported IgG, IgM levels decreased over time for both ocrelizumab and ofatumumab.
Mean/median percentage change from baseline in IgM levels: clinical trials of ocrelizumab and ofatumumab. CFB = change from baseline; CSF = cerebrospinal fluid; IgM = immunoglobulin M; LLN = lower limit of normal; OCR = ocrelizumab. Note: Changes in IgM levels are for summary purposes only; owing to heterogeneity in trial designs and outcomes, cross-trial comparisons should not be made. Detailed outcomes of clinical trials are presented in Appendix B, Supplemental Material. OBOE examined only CSF IgM levels and not serum IgM levels. ASCLEPIOS I/II: median IgM levels are presented; LLN for serum IgM defined as 0.4 g/L [17]. VELOCE: mean IgM levels are presented; LLN for serum IgM defined as 0.37 g/L [37]. OPERA I/II: mean IgM levels are presented; LLN for serum IgM defined as 0.4 g/L [35]. NCT00676715: mean IgM levels are presented. ASCLEPIOS I/II, APLIOS, APOLITOS, ALITHIOS pooled: mean IgM levels are presented; LLN for serum IgM defined as 0.4 g/L [29]. Treatment interruption due to notably low IgM levels (> 10% below LLN) and treatment discontinuation were reported for 9.1% and 3.3% of patients, respectively [17]
The OPERA I and II trials reported a consistent decrease in IgM levels from baseline through week 336, with a mean relative reduction of 55.8% at week 336 for all ocrelizumab participants combined [38] (Table S5). VELOCE reported a decrease in mean IgM levels from baseline to week 24 in the ocrelizumab treatment group, while IgM levels remained stable in the control group [19]. NCT00676715 reported a mean reduction of 34.87% in IgM with 3 cycles of ocrelizumab and 39.54% with 4 cycles of ocrelizumab at week 120 [22].
The ASCLEPIOS I and II trials reported a mean decrease of 30.9% at week 48 and 38.8% at week 96 for people treated with ofatumumab [41] (Fig. 4). Similarly, for the pooled data from the ASCLEPIOS I and II trials with data from the APOLITOS, APLIOS, and ALITHIOS studies, mean decreases of 31.8% and 46% were observed at week 48 and week 168, respectively, for participants treated with ofatumumab [17] (Fig. 4).
The 4 RWE studies reporting on IgM values studied ocrelizumab, and all reported a decrease in IgM levels over time with ocrelizumab treatment (Table S6). Lopez Ruiz et al. [28] reported that 11 of the 52 included participants had IgM levels < LLN at 78 weeks.
IgA
IgA data were reported for only 2 trial populations (Table S5). In the OPERA I and II trials, a decrease in mean IgA levels was observed for the ocrelizumab treatment arm over 336 weeks, and after a mean increase in the IFN β-1a arm up to week 96, a decrease was observed after the switch to ocrelizumab treatment [36, 39]. This trial also reported a change from baseline in IgA levels, with a mean 21.3% decline at week 264 reported for people treated with ocrelizumab. OPERA I/II was also the only trial to report the percentage of participants achieving a certain level for IgA. At baseline, 1.5% of participants had IgA levels < LLN in the ocrelizumab arm and 1.2% in the IFN β-1a–treated cohort. At the latest recorded timepoint of week 312, 7.5% of the ocrelizumab cohort and 3.9% of the IFN β-1a cohort had IgA levels < LLN [38]. IgA was not reported in clinical trials of ofatumumab but was reported in the OMS115102 dose-ranging study of high-dose intravenous ofatumumab [21]. In OMS115102, participants switching from placebo to ofatumumab 100 mg (considerably higher than the approved dose of 20 mg administered subcutaneously) had a mean change from baseline of − 0.07 (standard deviation [SD], 0.18) g/L at week 24 and − 0.00 (SD, 0.24) g/L at week 48. For those receiving placebo followed by ofatumumab 300 mg, mean change from baseline at week 24 was 0 (SD, 0.44) g/L, and 0.05 (SD, 0.21) g/L at week 48. Finally, for people receiving placebo then ofatumumab 700 mg, mean change from baseline was 0.49 (SD, 0.35) g/L at week 24 and 0.29 (SD, 0.26) g/L at week 48.
No data were provided on specific IgA levels in any of the included RWE studies. Evertsson et al. [26] reported that levels of IgA in blood were not affected by 52 weeks of ocrelizumab treatment based on the results of a retrospective study but presented no specific IgA values.
Association of Ig levels with infection
Three trial populations reported data on the association of Ig levels with infection: ASCLEPIOS I/II (trial population 1); ASCLEPIOS I/II, APLIOS, APOLITOS, ALITHIOS (trial population 2); and OPERA I/II, pooled with ORATORIO in most publications (trial population 3) (Table 3). Over all postbaseline visits, 14.2% of ASCLEPIOS I/II participants receiving ofatumumab had IgG below LLN. The proportion of ASCLEPIOS I/II participants on ofatumumab who experienced at least 1 infection within 1 month prior to and until 1 month after IgG below LLN was 27.6% (37 of 134; 3 serious) versus 50.6% (410 of 810) with IgG at or above LLN (21 serious) [29]. The proportion of participants on ofatumumab who experienced at least 1 infection within 1 month prior to and until 1 month after IgM below LLN was 31.1% (52 of 167; 2 serious), versus 51.5% (400 of 777) with IgM at or above LLN (18 serious) [29]. No association was observed with decreased IgM or IgG levels and increased risk of serious/nonserious infections in individuals treated with ofatumumab. Wiendl et al. [17] (ASCLEPIOS I/II, APLIOS, APOLITOS, and ALITHIOS trials) also reported on the association between IgG levels and infection risk and between IgM levels and infection risk for individuals undergoing long-term treatment with ofatumumab, concluding that no apparent association was observed between low IgG or IgM levels and risk of serious infections after 3.5 years of ofatumumab treatment. The OPERA I/II trials also reported data on the association of IgG, IgM, and IgA levels with infection. A numerical trend of lower rates of serious infections among higher quartiles of baseline IgG level was observed (by baseline IgG quartile, serious infections rates per 100 participant-years [95% CI] were as follows: Q1, 1.63 [0.95–2.61]; Q2, 1.55 [0.90–2.48]; Q3, 1.51 [0.86–2.45]; and Q4, 1.11 [0.57–1.94]) [37]. However, most data on association of Ig levels with infection rates reported for the OPERA I/II trials were pooled with the ORATORIO trial, which itself did not meet eligibility criteria for this SLR because it included people with PPMS. For IgG levels, 14 serious infections occurred during a drop in IgG level < LLN as compared with 208 serious infections for those during IgG levels ≥ LLN [35, 36]. The authors reported that an apparent association between decreased levels of IgG and rates of serious infections was observed, although the types, severity, duration, and outcomes of these infections were similar to those of the overall population treated with ocrelizumab and to the general MS population [35, 36]. For IgM levels, OPERA I/II reported that 71 serious infections occurred during a drop in IgM level < LLN as compared with 151 serious infections for those during IgM levels ≥ LLN [35, 36]. OPERA I/II were also the only trials to report data on the association of IgA levels with infection, with 7 serious infections occurring during a drop in IgA levels < LLN, compared with 215 when IgA levels were ≥ LLN [35].
Lopez Ruiz et al. [28] reported that no correlation between infection and Ig levels was found; however, the retrospective study may have had limited sample size to detect an association with 52 participants, with 7 experiencing infections, and Ig levels were reported for only 31 participants at baseline. None of the other included RWE studies reported data on the association of Ig levels with infection.
Discussion
This SLR aimed to identify data on Ig levels over time in people with RMS treated with ocrelizumab and ofatumumab, as well as the associations between Ig levels and infection risk, and the differences therein between those treated with ocrelizumab and ofatumumab in clinical trials and also RWE. Of the 30 publications included in the review, 24 reported on clinical trials (11 trials with results for 9 trial populations), and 6 reported on RWE studies. Ocrelizumab was the treatment of interest in 4 of the 9 trial populations and in all 5 of the RWE studies; ofatumumab was the treatment of interest in 5 of the 9 trial populations identified but none of the RWE studies. This discrepancy in the number of RWE studies included was to be expected because ocrelizumab was first approved in the US in 2017 and in Europe in 2018 and has been in widespread use since [1, 44], whereas ofatumumab was approved more recently, in 2020 and 2021, respectively, for treatment of RMS [6, 33]. A third anti-CD20 monoclonal antibody, ublituximab, was also in development for the treatment of RMS at the time of performing this SLR, but not yet approved and therefore not included in this review.
In trials and RWE studies evaluating ocrelizumab, IgG levels decreased in most studies identified. For instance, in the OPERA I and II trials, a decrease in mean IgG levels was observed in the ocrelizumab treatment arm, as well as after a switch to ocrelizumab in those who had been originally treated with IFN β [38]. Over a period of up to 7 years, serum IgG levels decreased at an average rate of 0.33 g/L per year (− 3% per year). At 312 weeks, IgG levels fell below the LLN in 7.7% of participants [38]. Mean IgG levels were generally stable in the VELOCE trial, with some fluctuation over time, and decreased slightly at 24 weeks from baseline (although it should be noted that this vaccine response study had a much shorter follow-up duration of only 24 weeks) [19]. Similar, albeit less consistent, patterns were also observed in the RWE studies: whereas most studies observed overall decreases in IgG levels over time with ocrelizumab treatment [25, 26, 28], some studies observed stable IgG levels or even slight increases in IgG levels from baseline [23, 24]. IgM levels appeared to decrease with ocrelizumab treatment. In the 1 ocrelizumab trial population for which IgA levels were reported, decreases in IgA levels were observed with ocrelizumab treatment and, interestingly, increasing IgA levels with IFN treatment [38]. In a retrospective RWE study, serum IgA levels were reportedly not affected by 52 weeks of treatment with ocrelizumab; however, no specific IgA values were provided.
Among ofatumumab trials, a transient reduction in IgG levels from baseline was observed in the ASCLEPIOS I and II trials until week 48, which stabilized by the end of follow-up [41]. When ASCLEPIOS I and II were further pooled with APOLITOS, APLIOS, and ALITHIOS, IgG levels also remained stable with up to 3.5 years of ofatumumab treatment [17]. These observations suggest that, in general, IgG levels do not diminish overall during ongoing treatment with ofatumumab in RMS, whereas IgG levels may be more likely to reduce over time with other B cell–depleting therapies, such as with ocrelizumab treatment. Among ofatumumab trials, a pattern of decreasing IgM levels with ofatumumab treatment was also generally observed, although most participants’ levels remained above LLN. In a dose-ranging study of intravenous ofatumumab, IgA remained stable [21].
Very few studies in people with RMS have reported an association between Ig levels with infection risk, in particular with respect to serious infections (which may have a more substantial impact on patients and on healthcare utilization). In the OPERA I/II trials, a numerical trend of lower rates of serious infections among higher quartiles of baseline IgG level was observed. Furthermore, when OPERA I/II data were pooled with those from the ORATORIO trial in PPMS, an association was observed between decreased IgG, IgM, and IgA levels and an increased risk of serious infection in ocrelizumab-treated participants, which was strongest for IgG and less pronounced for IgM or IgA [36]. It is to be noted that these data include participants who received any dose of ocrelizumab during the controlled treatment and associated open-label extension periods of the phase 3 OPERA and ORATORIO studies. Furthermore, these results must be interpreted cautiously given that they were observed in a combined population of people with RMS and people with PPMS, who in general tend to be older than those with RMS. The effect of age as a potential confounder affecting the relationship between Ig levels and infections may not be adequately accounted for. In the ASCLEPIOS I/II trials, no clear association was observed with decreased IgM or IgG levels and increased risk of serious/nonserious infections in participants treated with ofatumumab [6, 29]. Similarly, pooled analyses from the ASCLEPIOS I/II, APLIOS, APOLITOS, and ALITHIOS trials concluded that there was no apparent association between low IgG or IgM levels with risk of serious infection after 3.5 years of treatment with ofatumumab [17]; 4-year data from ALITHIOS presented after this SLR was conducted have shown consistent results [13]. No further information on the association of Ig levels with infection was reported across the remaining trials. Among real-world studies, Lopez Ruiz et al. [28] reported that no correlation between infection and Ig levels was found with ocrelizumab treatment. However, this retrospective study was small, potentially underpowered, very few people actually experienced infections, and Ig levels were only reported for a subset of the study cohort. Aside from this RWE study, none of the included RWE studies reported data on the association of Ig levels with infection.
Taken together, the results of this SLR suggest that ofatumumab therapy for people with RMS may have a more favorable effect than ocrelizumab therapy on IgG levels over time as observed in clinical trials, although it is important to note that no head-to-head trials have been conducted for these therapies. IgM decreases were seen with both ofatumumab and ocrelizumab therapy. There are a number of potential mechanisms for differences in IgG effects with ofatumumab and ocrelizumab. First, ofatumumab and other anti-CD20 monoclonal antibodies show differences in B cell depletion due to variations in epitope binding, avidity, and off rate [8, 45]. Ofatumumab, for example, binds to a unique CD20 epitope, attaching closer to the cell membrane than other monoclonal antibodies, potentially accounting for greater complement-dependent cytotoxicity and B cell lysis [8]. In addition, anti-CD20 monoclonal antibodies may have different biologic effects based on mechanism of action and route of administration (e.g., with ofatumumab administered at a lower dose and more frequently than ocrelizumab, and via subcutaneous injection), in turn leading to different subsets of B cells depleted and variations in time to B cell repletion [8].
It is important to consider these results within the context of the evidence base. The total duration of treatment was shorter for ofatumumab trials compared with ocrelizumab trials. Because IgG values decline very slowly over time, it is possible that an effect of ofatumumab on IgG has not yet been detected in trials. In addition, ofatumumab trials included a mandated treatment interruption for notably low IgG levels (> 20% below LLN) and notably low IgM levels (> 10% below LLN) [9], whereas in the ocrelizumab trials, treatment interruptions or discontinuations occurred based on low IgG levels but not low IgM levels. In the ofatumumab trials, the proportions of patients with treatment interruptions and discontinuations, respectively, were 0.1% and 0.2% for IgG levels and 9.1% and 3.3% for IgM levels [17]. Although in theory this might obscure potential IgG hypogammaglobinemia in the phase 3 ofatumumab core and extension trials, it is important to note that, for the majority of ofatumumab-treated patients, treatment interruptions were brief (< 2 months), potentially suggesting that there is a plausible biologic difference in effect of ofatumumab and ocrelizumab on IgG levels. Furthermore, results from 2 ocrelizumab trial populations, NCT00676715 [22] and OPERA I/II [38], respectively indicated a decrease in mean IgG level for ocrelizumab-treated patients at week 120 and increasing proportions of ocrelizumab-treated patients with IgG < LLN over time, up to week 168—the time points most comparable to those reported in the ofatumumab trials.
Importantly, the results of the RWE studies must be interpreted with caution; these studies are limited by smaller sample sizes, cohort differences, and heterogeneous testing methods, potentially contributing to the inconsistency in IgG observed across these studies as compared with clinical trial data. Furthermore, no RWE studies were identified for ofatumumab, and the evidence may evolve as such studies are conducted. In addition, lack of reporting of IgA levels across studies highlights a clear gap in knowledge that should be addressed in larger, future longitudinal studies. Few studies specified the types of infections they monitored, which may result in between-study variation due to differences in the incidence of certain types of infection across regions, pathogenicity of different infectious agents, and heterogenous, individual immune responses. These studies also did not report data on the evolution of infections or the stages of the primary and secondary immune responses. For instance, IgM provides an initial short-term response to a new infection before the onset of an IgG response, and IgA is more relevant in mucosal areas [46,47,48,49,50].
Limited evaluations of potential associations between Ig levels with infections and with serious infections, particularly among real-world studies, additionally highlights a further gap in the understanding and clinical relevance of Ig changes in the setting of B cell–depleting therapies among people with RMS. Data on the associations between Ig levels and infection for the OPERA I/II trials evaluating ocrelizumab were pooled with data from the ORATORIO trial, which included people with PPMS, and therefore are not comparable with results from trials conducted with RMS-only populations. Treatments with regulatory approval for RMS in the US and Europe at the time of this review were our focus; studies related to treatments that are used off label in clinical practice (e.g., rituximab) or that are not yet approved (e.g., ublituximab) were not evaluated. Recent RWE has shown that treatment with rituximab is associated with increased risk of hospitalization for COVID-19 relative to other DMTs [7]. There is a need for additional RWE on the relationships between Ig levels and rates and severity of infection for those with RMS and the impact of different treatments.
Some limitations of this review must be considered in the interpretation of our study findings. Because this study is an SLR, the methodology does not support cross-study comparisons; indirect comparison of aggregate data was not feasible owing to heterogeneity in the studies and a lack of common comparators, and therefore, we present only a narrative summary of the study results. While robust SLR methods were used in this review, and multiple databases and gray literature sources were searched, double screening was not used. Quality assessments were not conducted in this review and so no conclusions can be drawn on the quality of the studies reporting data. Some of the included publications were conference abstracts/posters and, therefore, reported limited detail. Additionally, some characteristics of the real-world studies, including small sample sizes, short durations of follow-up, and inconsistent definitions of infections, limit comparisons between these studies and the conclusions that can be drawn from them.
Conclusions
Results of this SLR suggest that in people with RMS, ofatumumab treatment might have a more favorable impact on IgG levels over time than ocrelizumab therapy, potentially as a result of these treatments’ respective mechanism of action and route of administration. There may therefore be an infection benefit risk associated with ofatumumab over ocrelizumab that warrants further study. IgM levels generally decrease with both ocrelizumab and ofatumumab treatment. Evidence from clinical trials indicates that Ig levels, and particularly IgG levels, may affect risk of infection in PwMS treated with ocrelizumab, ofatumumab, and other disease-modifying drugs. Additional long-term data are needed to further explore these findings.
References
Hauser SL, Cree BAC (2020) Treatment of multiple sclerosis: a review. Am J Med 133(12):1380–90.e2. https://doi.org/10.1016/j.amjmed.2020.05.049
Lassmann H, Brück W, Lucchinetti CF (2007) The immunopathology of multiple sclerosis: an overview. Brain Pathol 17(2):210–218. https://doi.org/10.1111/j.1750-3639.2007.00064.x
Hemmer B, Kerschensteiner M, Korn T (2015) Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol 14(4):406–419. https://doi.org/10.1016/S1474-4422(14)70305-9
Van Kaer L, Postoak JL, Wang C, Yang G, Wu L (2019) Innate, innate-like and adaptive lymphocytes in the pathogenesis of MS and EAE. Cell Mol Immunol 16(6):531–539. https://doi.org/10.1038/s41423-019-0221-5
Lovato L, Willis SN, Rodig SJ, Caron T, Almendinger SE, Howell OW et al (2011) Related B cell clones populate the meninges and parenchyma of patients with multiple sclerosis. Brain 134(Pt 2):534–541. https://doi.org/10.1093/brain/awq350
US Food and Drug Administration (2020) Kesimpta. Highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125326s070lbl.pdf. Accessed 16 August 2021
Spelman T, Forsberg L, McKay K, Glaser A, Hillert J (2022) Increased rate of hospitalisation for COVID-19 among rituximab-treated multiple sclerosis patients: a study of the Swedish multiple sclerosis registry. Mult Scler 28(7):1051–1059. https://doi.org/10.1177/13524585211026272
Bar-Or A, O’Brien SM, Sweeney ML, Fox EJ, Cohen JA (2021) Clinical perspectives on the molecular and pharmacological attributes of anti-CD20 therapies for multiple sclerosis. CNS Drugs 35(9):985–997. https://doi.org/10.1007/s40263-021-00843-8
Hauser SL, Cross AH, Winthrop K, Wiendl H, Nicholas J, Meuth SG et al (2022) Safety experience with continued exposure to ofatumumab in patients with relapsing forms of multiple sclerosis for up to 3.5 years. Mult Scler 28(10):576–1590. https://doi.org/10.1177/13524585221079731
Mayer L, Kappos L, Racke MK, Rammohan K, Traboulsee A, Hauser SL et al (2019) Ocrelizumab infusion experience in patients with relapsing and primary progressive multiple sclerosis: results from the phase 3 randomized OPERA I, OPERA II, and ORATORIO studies. Mult Scler Relat Disord 30:236–243. https://doi.org/10.1016/j.msard.2019.01.044
Disanto G, Ripellino P, Riccitelli GC, Sacco R, Scotti B, Fucili A et al (2021) De-escalating rituximab dose results in stability of clinical, radiological, and serum neurofilament levels in multiple sclerosis. Mult Scler 27(8):1230–1239. https://doi.org/10.1177/1352458520952036
Seery N, Sharmin S, Li V, Nguyen AL, Meaton C, Atvars R et al (2021) Predicting infection risk in multiple sclerosis patients treated with ocrelizumab: a retrospective cohort study. CNS Drugs 35(8):907–918. https://doi.org/10.1007/s40263-021-00810-3
Nicholas JA, Hauser SL, Cross AH, Winthrop K, Wiendl H, Meuth SG, et al (2022) Longer-term safety of ofatumumab in patients with relapsing multiple sclerosis. Presented at the Consortium of Multiple Sclerosis Centers Annual Meeting, National Harbor
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ et al (2022) Cochrane handbook for systematic reviews of interventions, version 6.3. www.training.cochrane.org/handbook. Accessed 30 November 2022
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 29(372):n71. https://doi.org/10.1136/bmj.n71
Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK et al (2020) Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med 383(6):546–557. https://doi.org/10.1056/NEJMoa1917246
Wiendl H, de Seze J, Bar-Or A, Correale J, Cross AH, Kappos L, et al. Effect of ofatumumab on serum immunoglobulin levels and infection risk in patients with relapsing multiple sclerosis over 3.5 years. Presented at the 37th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; October 13–15, 2021. Digital Experience
Weber M, Von Büdingen HC, Bar-Or A, Herman A, Harp C, Pei J et al (2020) Modulation of cerebrospinal fluid immunoglobulins by ocrelizumab treatment. Mult Scler J 26(3 Suppl):171–172. https://doi.org/10.1177/1352458520974937
Bar-Or A, Calkwood JC, Chognot C, Evershed J, Fox EJ, Herman A et al (2020) Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE study. Neurology 95(14):e1999–e2008. https://doi.org/10.1212/WNL.0000000000010380
Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B et al (2017) Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 376(3):221–234. https://doi.org/10.1056/NEJMoa1601277
GlaxoSmithKline. Ofatumumab dose-finding in relapsing remitting multiple sclerosis (RRMS) patients. ClinicalTrials.gov identifier: NCT00640328. Updated 11 April 2017. https://ClinicalTrials.gov/show/NCT00640328
Baker D, Pryce G, James LK, Marta M, Schmierer K (2020) The ocrelizumab phase II extension trial suggests the potential to improve the risk: benefit balance in multiple sclerosis. Mult Scler Relat Disord 44:102279. https://doi.org/10.1016/j.msard.2020.102279
Prezioso C, Grimaldi A, Landi D, Nicoletti CG, Brazzini G, Piacentini F et al (2021) Risk assessment of progressive multifocal leukoencephalopathy in multiple sclerosis patients during 1 year of ocrelizumab treatment. Viruses 13(9):1684. https://doi.org/10.3390/v13091684
van Lierop ZYGJ, Toorop AA, Coerver EME, Willemse EAJ, Strijbis EMM, Kalkers NF et al (2021) Ocrelizumab after natalizumab in JC-virus positive relapsing remitting multiple sclerosis patients. Mult Scler J Exp Transl Clin 7(2):20552173211013830. https://doi.org/10.1177/20552173211013831
Edgar N, Hoyt T, Foley J (2020) Can we predict ocrelizumab super responder status in relapsing remitting multiple sclerosis patients? Mult Scler J 26(3 Suppl):267. https://doi.org/10.1177/1352458520974937
Evertsson B, Hoyt T, Christensen A, Nimer FAL, Foley J, Piehl F (2020) A comparative study of tolerability and effects on immunoglobulin levels and CD19 cell counts with ocrelizumab vs low dose of rituximab in multiple sclerosis. Mult Scler J Exp Transl Clin 6(4):2055217320964505. https://doi.org/10.1177/2055217320964505
Evertsson B, Hoyt T, Christensen A, Al Nimer F, Foley J, Piehl F (2019) Comparative study of tolerability and effects on immunoglobulin levels and CD19 cell counts with ocrelizumab vs rituximab in multiple sclerosis. Mult Scler J 25:515–516. https://doi.org/10.1177/1352458519868080
Lopez Ruiz R, Eichau S, Guerra Hiraldo J, Dotor Garcia-Soto J, Ruiz de Arcos M, Ruiz-Pena J. Real world data on the use of ocrelizumab. Incidence of lymphopenia, B-cell and immunoglobulins evolution. Presented at the 37th Congress of the European Committee for Treatment and Research in Multiple Sclerosis; October 13–15, 2021. Digital Experience
Wiendl H, De Seze J, Bar-Or A, Correale J, Cross AH, Kappos L et al (2020) Serum immunoglobulin levels and infection risk in the phase 3 trials of ofatumumab in relapsing multiple sclerosis. Mult Scler J 26(3 Suppl):233–234. https://doi.org/10.1177/1352458520974937
de Seze J, Bar-Or A, Correale J, Cross AH, Kappos L, Selmaj K et al (2020) Effect of ofatumumab on serum immunoglobulin levels and infection risk in relapsing multiple sclerosis patients from the phase 3 ASCLEPIOS I and II trials. Eur J Neurol 27:1295–1296
de Seze J, Bar-Or A, Correale J, Cross AH, Kappos L, Selmaj K et al (2020) Effect of ofatumumab on serum immunoglobulin levels and infection risk in relapsing multiple sclerosis patients from the Phase 3 ASCLEPIOS I and II trials. Int J MS Care 22(s2). https://doi.org/10.7224/1537-2073-22.s2.1
Bar-Or A, De Seze J, Correale J, Cross A, Kappos L, Selmaj K et al (2020) Effect of ofatumumab on serum immunoglobulin levels and infection risk in relapsing multiple sclerosis (RMS) patients from the phase 3 ASCLEPIOS I and II trials (1300). Neurology 96(15 Supplement)
European Medicines Agency. Kesimpta summary of product characteristics. 24 June 2021. https://www.ema.europa.eu/en/documents/product-information/kesimpta-epar-product-information_en.pdf. Accessed 10 September 2021
Hauser SL, Kappos L, Arnold DL, Bar-Or A, Brochet B, Naismith RT et al (2020) Five years of ocrelizumab in relapsing multiple sclerosis: OPERA studies open-label extension. Neurology 95(13):e1854–e1867. https://doi.org/10.1212/WNL.0000000000010376
Derfuss T, Weber M, Hughes R, Eggebrecht J, Wang Q, Sauter A et al (2020) Serum immunoglobulin levels and risk of serious infections in the pivotal phase III trials of ocrelizumab in multiple sclerosis and their open-label extensions. Clin Neurophysiol 131(4):e196. https://doi.org/10.1016/j.clinph.2019.12.042. (Encore)
Derfuss T, Weber MS, Hughes R, Wang Q, Sauter A, Koendgen H et al (2019) Serum immunoglobulin levels and risk of serious infections in the pivotal Phase III trials of ocrelizumab in multiple sclerosis and their open-label extensions. Mult Scler J 25:20–21. https://doi.org/10.1177/1352458519868070
Bar-Or A, Bermel R, Weber MS, Hughes R, Lin CJ, Wang J et al (2020) Serum IG levels and risk of serious infections by baseline IG quartile in the pivotal phase III trials and open-label extensions of ocrelizumab in multiple sclerosis. Neurology 94(15 Suppl)
Hauser SL, Kappos L, Montalban X, Craveiro L, Chognot C, Hughes R et al (2021) Safety of ocrelizumab in patients with relapsing and primary progressive multiple sclerosis. Neurology 97(16):e1546-e1559. https://doi.org/10.1212/wnl.0000000000012700
US Food and Drug Administration. Ocrevus medical review(s). 5 November 2015. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761053Orig1s000MedR.pdf. Accessed 10 September 2021
European Medicines Agency. Ocrevus assessment report. Procedure No. EMEA/H/C/004043/0000. 9 November 2017. https://www.ema.europa.eu/en/documents/assessment-report/ocrevus-epar-public-assessment-report_en.pdf. Accessed 10 September 2021
European Medicines Agency. Kesimpta assessment report. Procedure No. EMEA/H/C/005410/0000. 28 January 2021. https://www.ema.europa.eu/en/documents/assessment-report/kesimpta-epar-public-assessment-report_en.pdf. Accessed 10 September 2021
US Food and Drug Administration. Kesimpta highlights of prescribing information. August 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125326s070lbl.pdf. Accessed 10 September 2021
US Food and Drug Administration. Ocrevus highlights of prescribing information. December 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761053s022lbl.pdf. Accessed 10 September 2021
European Medicines Agency. Ocrevus summary of product characteristics. 2018. Accessed 1 March 2022
Yu H, Graham G, David OJ, Kahn JM, Savelieva M, Pigeolet E et al (2022) Population pharmacokinetic-B cell modeling for ofatumumab in patients with relapsing multiple sclerosis. CNS Drugs 36(3):283–300. https://doi.org/10.1007/s40263-021-00895-w
Liu J, Wang Y, Xiong E, Hong R, Lu Q, Ohno H et al (2019) Role of the IgM Fc receptor in immunity and tolerance. Front Immunol 10:529. https://doi.org/10.3389/fimmu.2019.00529
Boes M (2000) Role of natural and immune IgM antibodies in immune responses. Mol Immunol 37(18):1141–1149. https://doi.org/10.1016/s0161-5890(01)00025-6
Keyt BA, Baliga R, Sinclair AM, Carroll SF, Peterson MS (2020) Structure, function, and therapeutic use of IgM antibodies. Antibodies (Basel) 9(4):53. https://doi.org/10.3390/antib9040053
Panda S, Ding JL (2015) Natural antibodies bridge innate and adaptive immunity. J Immunol 194(1):13–20. https://doi.org/10.4049/jimmunol.1400844
Schroeder HW Jr, Cavacini L (2010) Structure and function of immunoglobulins. J Allergy Clin Immunol 125(2 Suppl 2):S41-52. https://doi.org/10.1016/j.jaci.2009.09.046
Acknowledgements
The authors wish to thank Agnès Cuyàs and Frances Ramsey of RTI Health Solutions for their assistance with searching the literature databases and trial registries.
Funding
This study was funded by Novartis Pharmaceuticals Corporation. Medical writing support for manuscript development was provided by Kate Lothman, BA, of RTI Health Solutions, and infographic development support was provided by Julie Wilkinson, PhD, and Nancy Nguyen, PharmD, of Envision Pharma Group; funded by Novartis Pharmaceuticals Corporation. This manuscript was developed in accordance with Good Publication Practice (GPP3) guidelines. Authors had full control of the content and made the final decision on all aspects of this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Informed consent
Not applicable.
Competing interests
Dr. Shiv Saidha is a Professor in the Department of Neurology at the Johns Hopkins University School of Medicine. Dr. Saidha engaged in this research as a private consultant or advisor and not in his capacity as a Johns Hopkins faculty member, and has been compensated for a consulting or advising service by Novartis in income/honorarium. Dr. Saidha has received consulting fees from Medical Logix for the development of CME programs in neurology and has served on scientific advisory boards for Novartis, Biogen, Genentech Corporation, TG therapeutics, and Bristol Myers Squibb. He has performed consulting for Novartis, Genentech Corporation, JuneBrain LLC, and Lapix therapeutics. He is the PI of investigator-initiated studies funded by Genentech Corporation, Novartis, and Biogen. He previously received support from the Race to Erase MS foundation. He has received equity compensation for consulting from JuneBrain LLC and Lapix therapeutics. He was also the site investigator of a trial sponsored by MedDay Pharmaceuticals and is the site investigator of a trial sponsored by Novartis. Eric Maiese, Kerri Wyse, and Qiujun Shao are employees of Novartis Pharmaceuticals Corporation. Judith Bell, Sydney Harold, Jose Marcano Belisario, and Emma Hawe are employees of RTI Health Solutions and worked as consultants to Novartis Pharmaceuticals Corporation.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saidha, S., Bell, J., Harold, S. et al. Systematic literature review of immunoglobulin trends for anti-CD20 monoclonal antibodies in multiple sclerosis. Neurol Sci 44, 1515–1532 (2023). https://doi.org/10.1007/s10072-022-06582-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06582-y