Abstract
Purpose
Here , we aimed to assess the frequency and phenomenology of autonomic and neuropathic complaints of long-COVID and to evaluate them by means of electrophysiology.
Methods
Step 1. Patients with prior COVID-19 infection were screened by COMPASS-31 and mTORONTO to create the target population for further evaluation. Step 2. Patients with high scores were invited for a detailed history of their complaints and electrophysiological analysis, which included nerve conduction studies, cutaneous silent period (CSP), and sympathetic skin response (SSR). We also constituted a control group composed of healthy subjects of similar age and sex for electrophysiological analysis.
Results
There were 106 patients, who matched the study criteria. Among them, thirty-eight patients (%35.8) had neuropathic or autonomic complaints or both. Fatigue and headache were significantly more frequent in patients with autonomic and neuropathic complaints. Detailed examination and electrophysiological evaluation were performed in 14 of 38 patients. Neuropathic complaints were patchy and proximally located in the majority. The entire CSP suppression index was higher in the patients (p = 0.002). There was no difference in palmar and plantar SSR between patients and healthy subjects. mTORONTO scores were negatively correlated with palmar and plantar SSR amplitudes, and the correlation was moderate.
Conclusion
Neuropathic or autonomic complaints were seen in more than one-third of patients with long-COVID. Neuropathic complaints were generally patchy, proximally predominant, asymmetric, or diffuse. The CSP suppression index was abnormal whereas SSRs were normal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coronavirus disease-2019 (COVID-19) is a multi-system infection due to a categorically respiratory virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which had profound effects on every aspect of life since the declaration of a pandemic by the World Health Organization in March 2020. Although the SARS-CoV-2 shares similar mechanisms of invasion and action with the class of Betacoronavirus that it belongs to, the pandemic created new insights about the effects of the virus on humans regarding the acute and post-acute phases of the disease [1].
People with COVID-19 infection have a wide range of symptoms even after the end of the active period of the disease, the most common systemic symptoms being fatigue, headaches, and cognitive impairment (“brain fog”), followed by pain, weakness, and the symptoms suggestive of autonomic dysfunction such as orthostatic intolerance, palpitations, and gastrointestinal dysfunction [2]. The spectrum of these symptoms is called “long-COVID” which includes both ongoing symptomatic COVID-19 (from 4 to 12 weeks) and post-COVID-19 syndrome (12 weeks or more) [3]. Post-acute sequela of SARS-CoV-2 (PASC) is the alternative terminology that the US National Institutes of Health (NIH) suggests using, referring to the same spectrum of the symptoms except for the duration of illness required for the diagnosis which has not been specified.
Although the long-COVID seems to be a new entity developed with the pandemic, it overlaps with myalgic encephalomyelitis and chronic fatigue syndrome (ME/CFS) [4]. The diagnostic criteria of the ME/CFS include a reduction in the ability to perform occupational, educational, social, or personal activities; post-exertional malaise; and unrefreshing sleep, which are common in long-COVID. Either cognitive impairment or orthostatic intolerance is also required for ME/CFS, which emphasizes the autonomic involvement itself [5]. Although there are disputes regarding the actual existence of the entity due to the lack of objective diagnostic tools, which leaves the diagnosis based on the subjective complaints of the patients, it is still remarkable since it makes survivors of COVID-19 socially and professionally incapacitated similar to ME/CFS. In line with this, the World Health Organization has assigned an ICD code that corresponds to the post-COVID situation (https://www.who.int/standards/classifications/classification-ofdiseases/emergency-use-icd-codes-for-covid-19-disease-outbreak).
Autonomic and neuropathic complaints of long-COVID raise attention as their implications are highly debilitating among others. New-onset paresthesia and neuropathic pain in COVID-19 survivors were reported previously [6]. In a study examining 20 patients with long-COVID, nerve conduction studies were normal despite new-onset sensory disturbances in all patients [7]. When the autonomic dysfunction of long-COVID patients was evaluated, orthostatic intolerance and postural tachycardia syndrome were highlighted [8, 9]. Therefore, questions regarding the natural history and underlying pathophysiology of these symptoms in long-COVID wait to be answered [10]. Although it is not currently an agreed topic whether these symptoms belong to a central or peripheral nervous system pathology, studies that define and explore small fibers in long-COVID are being conducted as these symptoms might point out small fiber pathology. Accordingly, increased velocities of pupillary dilatation and baseline pupillary diameters along with a higher incidence of foot sudomotor dysfunction were shown in COVID-19 patients compared to healthy individuals in the acute phase [11]. Another study also showed that sweat function testing abnormality was observed in COVID-19 patients with sensory or autonomic symptoms in the chronic phase [12]. As well, skin biopsies of the long-COVID patients with new-onset paresthesia confirmed that small-fiber neuropathy might be possible [13]. Even so, electrophysiological techniques that demonstrate small-fiber dysfunction in long-COVID is needed, which can further elucidate the existence of small-fiber dysfunction after COVID-19 and supposedly the underlying pathophysiology.
Here, we aimed to perform electrophysiological studies evaluating sensory function and sudomotor function in a cohort of long-COVID patients. For this purpose, we screened all COVID-19 patients admitted to the outpatient clinics at least 4 weeks after the acute phase of the disease by screening tests that effectively capture sensory or autonomic involvement, and those with symptoms were invited for further neurological examination and electrophysiological studies, which included nerve conduction studies, cutaneous silent period (CSP), and sympathetic skin response (SSR). In this way, we aimed to disclose the percentage of these symptoms in long-COVID accurately and investigate the presence of sensory disturbances or autonomic symptoms more objectively. In a secondary analysis, we compared the clinical findings with findings in screening tests and electrophysiological findings, as well.
Patients and method
Patients
All consecutive patients, who were admitted to the outpatient clinic of the infectious disease department due to COVID-19 infection in the acute phase or hospitalized in the clinics for COVID-19 infection and returned for a follow-up visit between October and November 2021, were invited to complete the following scales at least 4 weeks after the acute phase. One of the investigators (Z.C.) followed the procedure.
Inclusion criteria were agreeing to participate after the purpose of the research and tests to be applied were explained, history of COVID-19 infection at least 4 weeks prior, and being > 18 years of age.
Exclusion criteria were being reluctant to participate after the purpose of the research and tests to be applied were explained, being < 18 years of age, having a disease that may cause small-fiber dysfunction (i.e., diabetes, amyloidosis, rheumatological diseases, history of drugs that are neurotoxic such as chemotherapy agents, lopinavir, daptomycin, linezolid), contraindications for electrophysiological investigations, having acute or recent infectious disease following the index COVID-19 infection that may cause similar symptoms (i.e., influenza, recurrent COVID-19, any febrile illness).
For step 2, we also included a control group composed of healthy subjects of similar age and sex.
The local ethical committee approved the study. All participants gave informed consent.
Study design
In the first step (step 1), one of the investigators (Z.C.) contacted all patients and sent the following scales online to patients who agreed to participate. Autonomic complaints were evaluated by the Composite Autonomic Symptom Score-31 (COMPASS-31), and neuropathic complaints were evaluated by the modified Toronto Neuropathy score (mTORONTO) [14, 15]. Abnormally high scores were designated as > 13 in COMPASS-31 [14] and > 4 in mTORONTO [15], and fatigue was evaluated by the fatigue severity scale (FSS) [16]. In the second step (step 2), an investigator (M.H.S.) checked the scores on the scales and invited the patients with abnormally high scores for further neurological examination and electrophysiological investigations.
Procedure
-
Step 1: Online evaluation
The patients who filled out the survey were questioned regarding the date of acute COVID-19 infection, duration of symptoms, anosmia, headache, myalgia, and questions of FSS, COMPASS-31, and mTORONTO. The date of acute COVID-19 infection was considered a positive PCR test, and the questionnaire was filled at least 4 weeks after a negative PCR test. The autonomic involvement of the patients was evaluated using the COMPASS-31, which normally consists of 31 questions divided into six areas: orthostatic intolerance, vasomotor symptoms, secretomotor symptoms, gastrointestinal symptoms, and bladder dysfunction and pupillomotor symptoms [14]. Questioning for neuropathic complaints was made according to the symptom subdivision of mTORONTO [15]. Fatigue was assessed by FSS [16].
-
Step 2: This step included two subitems: detailed clinical evaluation and electrophysiological investigations.
-
1. Clinical evaluation: Patients who had high scores in autonomic and neuropathic divisions of their survey were invited for further evaluation. During the evaluation, patients were asked about the natural course of the active period of their disease, as well as the post-infectious period regarding their symptoms. A detailed neurological examination was performed afterward. The demographic data and clinical information include other symptoms related to long-COVID such as weakness, sleep disorders, slowness in mental functions, and headache, along with chronic comorbidities, regularly used drugs, and medications due to COVID-19, were documented in detail
-
2. Electrophysiological investigations: All patients underwent standardized electrophysiological examination. Electrophysiological studies were performed by two investigators (A.G.), who were blind to the examination findings, using the Keypoint EMG device (Dantec, Denmark). We performed nerve conduction studies, followed by CSP and SSR.
-
Nerve conduction studies (NCS): We performed motor NCS of bilateral median and ulnar nerves in the upper extremities and bilateral peroneal and tibial nerves in the lower extremities using bar electrodes. The recording electrode for median motor NCS was on the abductor pollicis brevis muscle; the stimulation was done at the wrist and antecubital fossa. The recording electrode for ulnar motor NCS was on abductor digiti minimi muscle; the stimulation was performed at the wrist, cubital tunnel, and 6 cm proximal to cubital tunnel. The recording electrode for peroneal motor NCS was on the extensor digitorum brevis muscle, and the stimulation was at the ankle, 3 cm distal to the fibular head and 5 cm proximal to the fibular head. The recording electrode for tibial motor NCS was on flexor hallucis brevis muscle, and we stimulated behind the medial malleolus and popliteal fossa. The sensory NCS was performed bilaterally on the median and the ulnar nerves antidromically and unilaterally on the sural and superficial peroneal nerves. All recordings were done using supramaximal stimulations. All NCSs were performed by the methods for precautions of safety, measurements, and electrode placement [17]. The skin temperature was maintained at about 32 °C. Filter settings for sensory NCS were 20 Hz-2 kHz and for motor NCS 2 Hz-10 kHz. For sensory NCS, sweep speed was 2 ms/div, and sensitivity was 10 µV/div. For motor NCS, sweep speed was 5 ms/div, and sensitivity was 5 mV/div.
-
Cutaneous silent period (CSP): The CSP recordings were performed based on previous publications [18]. The CSP was recorded from the right abductor pollicis brevis muscle after painful stimulation of the digital nerves of the right index finger while the participants were performing a mild thumb abduction (at 25% of their maximal voluntary contraction) [19]. To control the level of muscle activity, the EMG signal was monitored by auditory and visual feedback. Filter settings were 30 and 10,000 Hz. Sweeps of 600 ms included a pre-stimulus period of 120 ms and a post-stimulus period of 480 ms. Ten consecutive traces were randomly recorded, rectified, and averaged in each condition and subject. The phases of CSP were calculated according to previous reports [20].
-
Sympathetic skin response (SSR): After skin cleansing, silver-silver chloride (Ag–AgCl) surface electrodes were placed using a conductive paste. The active one was on the palm of the foot, the reference was on the back of the hand or the foot, and the ground electrode was on the same extremity. Electrical stimulation was performed randomly with the aid of a stimulator on the skin to the median or tibial nerve at the opposite extremity. The duration of the stimulations was 0.1 ms, and the intensity was kept at 40 mA. The filter settings were set to 0.1 to 1,000 Hz, the sensitivity to 0.5 to 2 microvolts (mV) to division (div), and the scan time to 1 s/div. Obtained SSR latencies were calculated in milliseconds (ms) measured with the help of a cursor from where the first deflection started. The peak-to-peak distance of the deflection was calculated as amplitude as mV.
-
Data and statistical analysis
-
Step 1: Enrolled patients were stratified into groups with autonomic complaints only, with neuropathic complaints only, with autonomic and neuropathic complaints, and without autonomic/neuropathic complaints according to the symptoms defined by the questionnaires.
-
We first compared the following clinical features among these groups: age, sex, duration of symptoms, fatigue according to FSS, and myalgia.
-
Step 2: We measured the following parameters:
-
• For NCSs
-
o
The sensory nerve action potential and the compound muscle action potential amplitudes, motor nerve conduction velocities, distal latencies, and F-wave latencies
-
o
-
• For CSPs
-
o
CSP onset latency (I1 onset latency), CSP end latency (I2 end latency)
-
o
CSP duration, I1 duration, I2 duration
-
o
LLR onset latency (I1 end latency), LLR end latency (I2 onset latency), LLR duration, relative LLR amplitude
-
o
Total CSP suppression index, I1 suppression index, I2 suppression index
-
o
Post-CSP excitation index
-
o
-
• For SSRs
-
o
Amplitude and normalized latency according to the length of upper/lower extremity
-
o
First, we compared these findings between patients and healthy subjects. We also compared electrophysiological findings among groups with autonomic complaints only, neuropathic complaints only, autonomic and neuropathic complaints, and healthy subjects.
Data analyses were performed using the SPSS 22.0 software statistical package. The data distribution was not normal.
In step 1, the comparisons were made by the Kruskal–Wallis test for quantitative data. Post-hoc analysis was done by the Mann–Whitney U test. We used the Chi-square test for qualitative data.
In step 2, the comparisons between patients and healthy subjects were made by the Mann–Whitney U test. We used the Chi-square test for qualitative data. We also classified patients according to neurological findings and made a second comparison. For this comparison, we used the Kruskal–Wallis test, and post hoc analysis was done by the Mann–Whitney U test.
Correlations between abnormal electrophysiological findings and mTORONTO and COMPASS-31 scale were done using Pearson’s correlation analysis.
A p-value ≤ 0.05 was considered significant.
Results
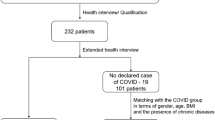
During the study period, we were able to contact all COVID-19 patients. We sent the questionnaires to those who were eligible (n = 172 patients), and 106 of them fulfilled the questionnaire online. The mean age was 39.4 ± 12.5, and there were 47 (44.3%) women. The mean duration from the negative PCR test to the time of response to the questionnaire was 147 days (median: 104 days, ranges 31–390 days).
Among these 106 patients, 18 (16.9%) had comorbid conditions: hypothyroidism and thyroid replacement therapy (n = 4); diabetes mellitus with oral antidiabetic medications (n = 4); essential hypertension and use of amlodipine (n = 2); warfarin anticoagulation for previous valve replacement (n = 1); psoriasis with no regularly used drugs (n = 1); asthma with inhaler steroids (n = 1); use of trazodone and paroxetine for insomnia and anxiety (n = 2); history of renal transplantation and use of ramipril, tacrolimus, prednisolone, and mycophenolic acid (n = 1); lymphoproliferative diseases (n = 2).
Findings in Step 1
Thirty-eight patients (%35.8) had one or both of the complaints (neuropathic or autonomic). Thirteen patients had high mTORONTO scores (the group with neuropathic complaints only), eight had high COMPASS-31 scores (the group with autonomic complaints only), and 17 had both high mTORONTO scores and high COMPASS-31 scores (the group with autonomic and neuropathic complaints). The median (minimum–maximum) values of COMPASS-31 subdomain scores were as follows: orthostatic intolerance 8 (0–24), vasomotor 0 (0–2.5), secretomotor 2.1 (0–10.7), gastrointestinal 3.6 (0–16.1), urinary 1.1 (0–4.4), and pilomotor 0 (0–0).
Age, gender, and duration were similar between groups (Table 1).
The number of patients with anosmia was not different among groups (p = 0.146). Thirty (28.3%) patients had a headache, and there was a significant difference among groups (p < 0.001). Headache was more frequent in the group with both autonomic and neuropathic complaints compared to the group without autonomic/neuropathic complaints or the group with neuropathic complaints (p < 000.1 and p = 0.003, respectively).
There was a significant difference among groups regarding FSS. A post hoc analysis showed that the group with autonomic complaints, the group with neuropathic complaints, and the group with both autonomic and neuropathic complaints had higher FSS scores compared to the group without autonomic/neuropathic complaints (with neuropathic complaints vs without autonomic/neuropathic complaints p = 0.021; with autonomic complaints vs without autonomic/neuropathic complaints p = 0.008; with both neuropathic and autonomic complaints vs without autonomic/neuropathic complaints p = 0.005).
Among the 38 patients, detailed examination and electrophysiological evaluation were performed for 14. For those 24 patients who could not be further evaluated, the reasons were as follows: one patient was pregnant, three patients had active influenza infection after COVID-19, two patients had an active lymphoproliferative disease, and 18 were unwilling to participate. At this step, there were patients with psoriasis, hypothyroidism, renal transplantation, valvular replacement, and asthma. Among these patients, we excluded two patients with lymphoproliferative diseases, because they had complaints and medical files suggesting peripheral nerve disorders in the history. We did not exclude other patients because history and medical files did not suggest any symptoms or signs of peripheral nervous system disorder before COVID-19.
The main reason for the unwillingness of patients was expressed as improvement of their symptoms during the time lapse between the survey and evaluation appointment or anxiety to come to the hospital and have COVID-19 infection again. Therefore, detailed history and neurological examination along with the NCS, CSP, and SSR were performed on the remaining 14 patients. Figure 1 shows the flow diagram of the study.
Findings in Step 2
-
1.
Clinical findings: Among patients who underwent detailed neurological examination, all except one experienced COVID-19 infection at home. The only patient who was hospitalized did not require invasive ventilation or intensive care unit, but she needed O2 support constantly during hospitalization. Five patients had chronic illnesses which were psoriasis, hypothyroidism, renal transplantation, valvular replacement, and asthma. Three of 14 patients complained of headaches; they all had prior headache history. Thirteen patients described new-onset sensory complaints, which occurred during long-COVID. These complaints were expressed as tingling, prickling, pain, burning, and sensitivity to touch. Figure 2 demonstrates illustrations of sensory complaints. We were able to classify patients according to the localization of sensory symptoms under eight groups. All had normoactive deep tendon reflexes and normal sensory examination. Eleven of 14 patients also had autonomic complaints, the most prominent of them being orthostatic intolerance. Complaints about memory disturbances, sleep problems, and fatigue were common among these 14 patients. Detailed clinical characteristics of 14 patients are listed in Table 2.
Topographic patterns of sensory symptoms in patients with long-COVID. A Proximal-predominant (patient 10/13). B Upper limb proximally predominant (patient 1/12). C Distal predominant (patient 4/5). D Diffuse (“whole body”) (patient 2/7). E Upper body predominant diffuse (patient 9). F Cape-like distribution on backside of body and upper limbs (patient 8). G Asymmetric (patient 11). H Lower limb proximally predominant (patient 6/14)
Electrophysiological findings
-
Nerve conduction studies: The NCSs were normal in all participants except for mild carpal tunnel syndrome (CTS) in one patient.
-
Cutaneous silent period (CSP): The CSP was obtained in all patients except one with CTS. Compared to healthy individuals, the entire CSP suppression index was higher in the patients (p = 0.002). The I1 suppression index and I2 suppression index were also higher in patients than those in healthy subjects (p = 0.040 and p = 0.004; respectively). The onset and end latencies as well as the duration of CSP were not different (Table 3).
When we compared patients with different neurological findings (groups with autonomic complaints only, with neuropathic complaints only, and with autonomic and neuropathic complaints), there was a significant difference in the entire CSP suppression index and I2 suppression index. Post hoc analysis disclosed that these parameters were significantly higher in the group with autonomic complaints only compared to the group with autonomic and neuropathic complaints (p = 0.010 and p = 0.014, respectively).
COMPASS-31 or mTORONTO scores did not correlate with any of the CSP parameters.
-
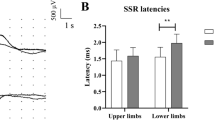
Sympathetic skin response (SSR): Both palmar and plantar SSRs were obtained in all patients. Latencies and amplitudes were similar between patients and healthy individuals (Table 3).
There was no difference among groups with autonomic complaints only, neuropathic complaints only, and autonomic and neuropathic complaints regarding SSR latency or amplitude.
mTORONTO scores negatively correlated with palmar and plantar SSR amplitudes, and the correlation was moderate (r = − 0.583, p = 0.037 and r = − 0.679, p = 0.022, respectively).
Discussion
In this study, we demonstrated that neuropathic or autonomic complaints were seen in more than one-third of patients after COVID-19 infection, and these complaints were commonly associated with fatigue and headache. Neuropathic complaints were generally patchy, proximal predominant, asymmetric, or diffuse. The CSP suppression index was abnormal whereas SSRs were normal.
Almost 10% of COVID-19 patients are candidates for long-COVID [7]. In a study conducted by questioning, the related symptoms in 4755 patients with a history of hospitalization due to COVID-19, its incidence was found to be 56% in the 6 to 8 months following the infection [21]. In another study, it was 76% with shorter follow-up periods (maximum 60 days) [13]. Neuromuscular symptoms are common during the active period of the disease, and it seems that they are also common in long-COVID. Any part of the peripheral nervous system is affected by long-COVID, being the most common fatigue [22]. However, autonomic dysfunction is also common. It is demonstrated that dysautonomia plays an important role in the acute phase of COVID-19 patients even if non-critically ill [23]. A retrospective review of symptoms concerning para-/post-infectious autonomic dysfunction after COVID-19 revealed lightheadedness, orthostatic headache, syncope, hyperhidrosis, and burning pain [24]. The symptoms occurred for up to 4 months. The patients in our study reported similar symptoms. The duration of symptoms was as long as 390 days. The association between headache, fatigue, and autonomic symptoms was striking. These studies generally reported sudomotor dysfunction along with abnormal symptoms [11, 12]. Although five patients in our study reported symptoms suggesting secretomotor dysfunction, we were unable to show abnormal SSRs. The main methodological difference between the previous studies from our study was that they created a study population from patients who were admitted to the neurology outpatient clinics. Therefore, these patients could have had a more severe form of involvement. An interesting finding was the negative correlation between sudomotor functions of the skin and neuropathic symptom severity, i.e., more severe neuropathic symptoms, and more reduction in the sudomotor function of the skin [27].
In literature, there are patient reports with large-fiber neuropathy and even immune neuropathy in the long-COVID period [26]. However, the nerve conduction studies in our patients were normal. Symptoms, mTORONTO, and COMPASS-31 scores of 14 patients suggested that patients in this cohort probably had small-fiber neuropathy. However, only one among them had symptoms compatible with length-dependent distal small-fiber neuropathy (patient 4). As seen in the illustrations, other patients had patchy, proximally predominant, asymmetric, or diffuse sensory complaints. These findings illustrated here were quite similar to what was described by Gemignani et al. [27] suggesting a non-length–dependent small-fiber neuropathy. This raises the question of whether COVID-19 infection leads to an inflammatory reaction in the dorsal root ganglion since non-length–dependent small-fiber neuropathy develops secondary to a lesion in the dorsal root ganglion. The type of lesion is generally autoimmune if there is small fiber type involvement in the dorsal root ganglion [28]. Abrams et al. [13] performed skin biopsies in patients with similar symptoms and described non-length–dependent small-fiber neuropathy in half and length-dependent small-fiber neuropathy in the remaining half. Keeping in mind CSP and SSR were relatively normal in these patients and one of the main limitations of our study was the lack of skin biopsy, we suggest that some patients with long-COVID may be affected by a non-length–dependent small-fiber neuropathy.
The onset latency of CSP in patients was comparable to that in healthy subjects. The onset latency reflects the functions of A-delta fibers [29]. We may speculate that A-delta fibers are operating smoothly in the distal parts of the presented patients. However, there is an abnormal modulation of CSP reflected by abnormal suppression indices [30]. In previous studies, suppression was reduced in upper motoneuron involvement in primary lateral sclerosis or amyotrophic lateral sclerosis with predominant upper motor neuron involvement [31] or by the effect of remote muscle vibration [32]. Assuming normal CSP onset latency suggests normal A-delta functioning in the relevant segment, the abnormally less suppression is probably related to abnormal modulation, which may be secondary to the fatigue or associated central nervous system findings in these patients. In non-length–dependent small-fiber neuropathy, loss of intraepidermal nerve fibers is more prominent on more proximal parts of the body with relative sparing on more distal parts [33], probably also sparing A-delta functions on distal parts. The comorbid conditions or drugs used by patients have not yet been reported to affect CSP [34, 35].
There were certain limitations of the study. As mentioned above, we did not perform skin biopsy or some of the electrophysiological tests. Other studies reported comorbid disorders with small-fiber neuropathy in long-COVID; however, we excluded such patients by step 2 to analyze the concrete effect of COVID-19 on small fibers. Another limitation of the study was the inability to assess the patients without autonomic or neuropathic complaints, as these asymptomatic COVID-19 survivors rejected hospital admissions due to the pandemic conditions.
In conclusion, small-fiber dysfunction, reflected by neuropathic and autonomic complaints, is common in long-COVID. Neuropathic complaints are dominated by patchy and proximal involvement. Abnormal suppression of CSP suggests abnormal modulation of the nociceptive pathways.
References
Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, Duan G (2020) Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses 12:372
Larsen NW, Stiles LE, Miglis MG (2021) Preparing for the long-haul: autonomic complications of COVID-19. Auton Neurosci 235:102841
(2020) COVID-19 rapid guideline: managing the long-term effects of COVID-19. National Institute for Health and Care Excellence (NICE), London
Wong TL, Weitzer DJ (2021) Long COVID and Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS)-a systemic review and comparison of clinical presentation and symptomatology. Medicina (Kaunas) 57:418
Lim EJ, Son CG (2020) Review of case definitions for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med 18:289
Munblit D, Bobkova P, Spiridonova E, et al (2021) Incidence and risk factors for persistent symptoms in adults previously hospitalized for COVID-19 51:1107–1120
Agergaard J, Leth S, Pedersen TH, Harbo T, Blicher JU, Karlsson P, Østergaard L, Andersen H, Tankisi H (2021) Myopathic changes in patients with long-term fatigue after COVID-19. Clin Neurophysiol 132(8):1974–1981. https://doi.org/10.1016/j.clinph.2021.04.009
Murga I, Aranburu L, Gargiulo PA, Gómez Esteban JC, Lafuente JV (2021) Clinical heterogeneity in ME/CFS A way to understand long-COVID19 fatigue. Front Psychiatry 12:735784
Buoite Stella A, Furlanis G, Frezza NA, Valentinotti R, Ajcevic M, Manganotti P (2022) Autonomic dysfunction in post-COVID patients with and witfhout neurological symptoms: a prospective multidomain observational study. J Neurol 269:587–596
Raj SR, Arnold AC, Barboi A, Claydon VE, Limberg JK, Lucci VM, Numan M, Peltier A, Snapper H, Vernino S (2021) American Autonomic Society. Long-COVID postural tachycardia syndrome: an American Autonomic Society statement. Clin Auton Res 31:365–368
Bellavia S, Scala I, Luigetti M, Brunetti V, Gabrielli M, Zileri Dal Verme L, Servidei S, Calabresi P, Frisullo G, Della Marca G (2021) Instrumental evaluation of COVID-19 related dysautonomia in non-critically-ill patients: an observational, cross-sectional study. J Clin Med 10:5861
Hinduja A, Moutairou A, Calvet JH (2021) Sudomotor dysfunction in patients recovered from COVID-19. Neurophysiol Clin 51:193–196
Abrams RMC, Simpson DM, Navis A, Jette N, Zhou L, Shin SC (2022) Small fiber neuropathy associated with SARS-CoV-2 infection. Muscle Nerve 65:440–443
Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W (2012) COMPASS 31: a refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin Proc 87:1196–1201
Bril V, Tomioka S, Buchanan RA, Perkins BA, the mTCNS Study Group (2009) Reliability and validity of the modified Toronto Clinical Neuropathy Score in diabetic sensorimotor polyneuropathy. Diabet Med 26:240–246
Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD (1989) The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol 46:1121–1123
Kimura J (2006) Electrodiagnosis in diseases of nerve and muscle. Principles and practice Oxford University Press, New York
Kofler M (2003) Functional organization of exteroceptive inhibition following nociceptive electrical fingertip stimulation in humans. Clin Neurophysiol 114:973–980
Kofler M, Kumru H, Stetkarova I, Schindler C, Fuhr P (2007) Muscle force up to 50% of maximum does not affect cutaneous silent periods in thenar muscles. Clin Neurophysiol 118:2025–2030
Kumru H, Opisso E, Valls-Solé J, Kofler M (2009) The effect of a prepulse stimulus on the EMG rebound following the cutaneous silent period. J Physiol 587:587–595
Bell ML, Catalfamo CJ, Farland LV et al (2021) Post-acute sequelae of COVID-19 in a non-hospitalized cohort: results from the Arizona CoVHORT. PLoS One 16:e0254347
Shah W, Hillman T, Playford ED, Hishmeh L (2021) Managing the long term effects of covid-19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ 372:n136
Scala I, Bellavia S, Luigetti M et al (2022) Autonomic dysfunction in non-critically ill COVID-19 patients during the acute phase of disease: an observational, cross-sectional study. Neurol Sci 24:1–9
Shouman K, Vanichkachorn G, Cheshire WP, Suarez MD, Shelly S, Lamotte GJ, Sandroni P, Benarroch EE, Berini SE, Cutsforth-Gregory JK, Coon EA, Mauermann ML, Low PA, Singer W (2021) Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res 31:385–394
Kucera P, Goldenberg Z, Kurca E (2004) Sympathetic skin response: review of the method and its clinical use. Bratisl Lek Listy 105:108–116
Oaklander AL, Mills AJ, Kelley M, Toran LS, Smith B, Dalakas MC, Nath A (2022) Peripheral neuropathy evaluations of patients with prolonged long COVID. Neurol Neuroimmunol Neuroinflamm 9:e1146
Gemignani F, Bellanova MF, Saccani E, Pavesi G (2022) Non-length-dependent small fiber neuropathy: not a matter of stockings and gloves. Muscle Nerve 65:10–28
Mota IA, Fernandes JB, Cardoso MN, Sala-Blanch X, Kofler M, Valls-Solé J (2015) Temporal profile of the effects of regional anesthesia on the cutaneous reflexes of foot muscles. Exp Brain Res 233:2587–2596
Serrao M, Parisi L, Pierelli F, Rossi P (2001) Cutaneous afferents mediating the cutaneous silent period in the upper limbs: evidences for a role of low-threshold sensory fibres. Clin Neurophysiol 112:2007–2014
Shefner JM, Logigian EL (1998) The mixed nerve silent period in normal subjects and patients with amyotrophic lateral sclerosis. Electromyogr Clin Neurophysiol 38:505–510
Castro J, Swash M, de Carvalho M (2021) The cutaneous silent period in motor neuron disease. Clin Neurophysiol 132:660–665
Aydın Ş, Kofler M, Bakuy Y, Gündüz A, Kızıltan ME (2019) Effects of vibration on cutaneous silent period. Exp Brain Res 237:911–918
Provitera V, Gibbons CH, Wendelschafer-Crabb G, Donadio V, Vitale DF, Loavenbruck A, Stancanelli A, Caporaso G, Liguori R, Wang N, Santoro L, Kennedy WR, Nolano M (2018) The role of skin biopsy in differentiating small-fiber neuropathy from ganglionopathy. Eur J Neurol 25:848–853
Kofler M, Leis AA, Valls-Solé J (2019) Cutaneous silent periods - part 1: update on physiological mechanisms. Clin Neurophysiol 130:588–603
Kofler M, Leis AA, Valls-Solé J (2019) Cutaneous silent periods - part 2: update on pathophysiology and clinical utility. Clin Neurophysiol 130:604–615
Acknowledgements
We thank the healthcare givers all around the world who have a heroic battle against the unknown, especially in the early periods of the pandemic.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Approval was obtained from the ethics committee of Istanbul University Cerrahpasa- Cerrahpasa Medical Faculty. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ser, M.H., Çalıkuşu, F.Z., Tanrıverdi, U. et al. Autonomic and neuropathic complaints of long-COVID objectified: an investigation from electrophysiological perspective. Neurol Sci 43, 6167–6177 (2022). https://doi.org/10.1007/s10072-022-06350-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-022-06350-y