Abstract
Introduction
Neurological sequelae following SARS-CoV-2 infection still represent a serious concern both for neurologists and neuroscientists. In our paper, we investigated pain, myalgia, and fatigue as symptoms in long-COVID patients with an electrophysiological approach, comprising the evaluation of sympathetic skin responses (SSRs) and quantitative electromyography (qEMG).
Materials and methods
Twelve patients were enrolled (mean age, 47.7 ± 11.6 years), referred to our attention because of myalgia, pain, or muscle cramps, which persisted about 6 months after the diagnosis of SARS-CoV-2 infection. They underwent conventional electroneurography (ENG), needle electromyography (EMG), and SSRs; moreover, qEMG was performed by sampling at least 20 motor unit potentials (20–30 MUPs) during weak voluntary contraction in deltoid and tibialis anterior muscles. The mean duration, amplitude, and percentage of polyphasic potentials were assessed and compared with healthy and age-matched volunteers.
Results
ENG did not disclose significant changes compared to healthy subjects; needle EMG did not reveal denervation activity. In addition, qEMG showed MUPs similar to those recorded in healthy volunteers in terms of polyphasia (deltoid: p = 0.24; TA: p = 0.35), MUP area (deltoid: p = 0.45; TA: p = 0.44), mean duration (deltoid: p = 0.06; TA: p = 0.45), and amplitude (deltoid: p = 0.27; TA: p = 0.63). SSRs were not recordable from lower limbs in seven patients (58%) and from the upper ones in three of them (25%).
Conclusion
Our data suggest an involvement of the autonomic system, with a focus on cholinergic efferent sympathetic activity, without any evidence of myopathic changes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is well known that SARS-CoV-2 leads to several neurological manifestations, including large-vessel strokes, seizures at onset, muscle pain, and para-infectious neuropathies. The neurological involvement is due to different mechanisms of action, probably comprising a direct spread through nerve terminals, as well as a disruption of the blood–brain barrier (BBB), as recently suggested by neuropathological studies in humans [1,2,3,4]. Moreover, following the acute phase of the illness, both neurological and non-neurological sequelae occur frequently: they are usually defined as “long-COVID syndrome” and comprise a number of heterogeneous complications, ranging from pain to smell disturbances and cognitive impairment, especially in the domains of processing speed and long-term visuo-spatial and verbal memory [5]. These complications often last for months, and their duration does not correlate with the severity of the primary infection [6].
Among these, recent papers have focused the attention on pain, fatigue, and muscle symptoms, suggesting a combination of central and peripheral mechanisms and highlighting the role of a reduced intracortical GABAergic inhibition underlying their occurrence [7]. Nonetheless, little is known about the neurophysiology of muscle and peripheral nerve involvement in long-COVID patients [8,9,10]; in particular, it is not clear whether pain, a frequent complication of SARS-CoV-2 infection, is related to the impairment of either peripheral nerve terminals or muscle fibers. Such information could significantly impact not only the prognosis but also the choice of pharmacological and non-pharmacological treatments, guiding rehabilitative interventions, which are still unspecific and refer to protocols used for long-COVID–like symptoms [11].
In this paper, we neurophysiologically evaluated patients with “long-COVID syndrome” referred to our laboratory by performing both qualitative and quantitative electromyography (EMG) and assessing small fiber involvement.
Materials and methods
Patients and clinical assessment
Twelve patients (mean age, 47.7 ± 11.6 years; eight women) were enrolled in the study (Table 1). The recruitment took place during the outpatient activity, and each patient was referred to our hospital because of myalgia, pain, or muscle cramps, which persisted about 6 months after the diagnosis of SARS-CoV-2 infection (mean duration of symptoms from the infection, 175.3 ± 31.0 days). Diagnosis of COVID-19 was confirmed by positive results on a reverse-transcriptase-polymerase-chain-reaction (RT-PCR) assay performed on nasopharyngeal and throat swab. None of them was hospitalized because of COVID-19, and only three patients were treated with low-dosage steroids during the acute phase of the illness. None of them reported the assumption of drugs interfering with the neuromuscular transmission at the time of the neurophysiological assessment. Common causes of neuropathies were also excluded by a preliminary interview (i.e., diabetes, dysthyroidism, vitamin B12 and/or folate deficiency, neoplastic/paraneoplastic syndromes). Other exclusion criteria were a pre‐existing neuromuscular transmission failure, polyradiculoneuropathies, and central nervous system disorders.
Each patient was asked about physical fatigue, myalgia, joint pain, and muscle cramps. Both sensory and muscle symptoms are reported in Table 1. All patients were vaccinated before entering the study, with either two or three doses (Table 1).
Creatine-kinase (CK) serum levels were within normal limits in all patients (132 ± 44.7 U/L; normal values, < 210 U/L).
Electroneurography (ENG) and conventional electromyography (EMG)
Neurophysiological parameters were compared with those recorded from 15 healthy and age-matched volunteers (10 women, 49.3 ± 8.9 years). Each patient underwent nerve conduction studies, including a bilateral examination of the median, ulnar, deep peroneal (from the extensor digitorum brevis muscle, EDB) and tibial (abductor hallucis brevis, AH) motor nerves (compound motor action potentials, CMAPs) and of the median, ulnar, radial, and sural sensory nerves (sensory action potentials, SAPs). Abductor digiti minimi muscle (ADM) responses to 3 Hz repetitive stimulation of the ulnar nerve were performed to rule out a neuromuscular transmission disease, at rest (T0), immediately after 60″ contraction (T1), 1 (T2), and 3 min (T3) later. Changes in CMAP sizes are quantified by calculating the percentage change in negative peak amplitude between the first and the fourth responses.
Electromyographic signal was captured by needle electrodes and analyzed (band-pass 10 Hz–10 kHz; sampling rate, 5 kHz; sensitivity set, 200 µV/Div; sweep speed, 10 ms/Div).
Evaluation of small fibers
Sympathetic skin responses (SSRs) were evaluated in all patients and recorded from the most affected side, either left or right, then compared with those obtained from 15-age- and sex-matched volunteers.
The stimulator was placed over a median nerve at the wrist, and the stimulation intensity was set at 20 mA (duration, 0.2 ms). Surface electrodes were positioned on palms and soles with reference electrodes placed over the dorsal region [12]. The filter was set at 0.5–2000 Hz. For each limb, three recordings were performed with an inter-stimulus interval between 20 and 30 s to avoid habituation. The response was considered not recordable when no change larger than 50 mV was observed in any of the three recordings in 2 s following a stimulus [12, 13].
Quantitative electromyography (qEMG)
Qualitative electromyography (EMG) of both deltoid and anterior tibial muscles was performed in healthy controls and patients. Additionally, EMG of other muscles was performed in patients with relevant symptoms [8]. EMG was performed using a concentric 35-mm Dantec needle electrode and the Department́ standard filter settings of 20 Hz–10 kHz, gain (100 mV/division), and sweep speed (10 ms/division). The presence of either denervation (fibrillation potentials and positive sharp waves) or spontaneous activity (e.g., fasciculations) was assessed at three separate sites per muscle, and spontaneous activity at more than two sites was required for abnormality [14]. Rest activity was monitored for at least 60″ at each insertion point.
Quantitative motor unit potential (MUP) analysis was done by sampling at least 20 MUPs (20–30 MUPs) during weak voluntary contraction. The method for multi-MUPs analysis is described in detail elsewhere [15,16,17]. In brief, the operator determines when the computer is to analyze about 30 s of a previously acquired signal. MUPs are selected and extracted (signal decomposition) according to software definitions and then analyzed by using different algorithms. MUPs are then sorted into classes, each representing the average of the selected MUPs.
The mean duration, amplitude, and percentage of polyphasic (i.e., five phases or more) potentials were assessed [17]. A mean MUP duration lower than the 95% confidence interval of healthy controls was considered as myopathic. This method is now considered the most reliable, fastest, and easiest to perform among three different quantitative EMG methods proposed so far; it usually requires about 5 min per muscle to be performed [18].
EMG interpretation was completed by 2 trained neurophysiologists (T.B.; L.C.), and qEMG was performed on the most affected side, either left or right.
The whole electrophysiological protocol required about 45 min for each patient.
Statistical analysis
Parametric analyses were used, as all datasets passed the Shapiro–Wilk test for normality (p > 0.05). Neurophysiological data were compared between patients and age-matched volunteers by using an unpaired t-test (p < 0.05). Data were analyzed using SPSS v. 21.0 for Windows (SPSS Inc.).
Results
Electroneurography (ENG) and conventional electromyography (EMG)
ENG and EMG were unremarkable in each patient. None of them showed spontaneous activity (i.e., fibrillation potentials) in any muscle (Table 2), and voluntary contraction showed an interference pattern of activation. Repetitive nerve stimulation (RNS) showed only a mild decrease of CMAP amplitudes until T2 (T0: − 3.4 ± 2.1; T1: − 5.1 ± 1.6; T2: − 2.5 ± 0.9; T3: 4.7 ± 3.3), falling within the normal range as extensively reported in literature [19, 20].
Small fiber involvement
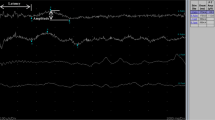
SSRs were not recordable from lower limbs in seven patients (58%) and also from the upper ones in four of them (25%; patients 1, 4, 5, and 11, as reported in Table 1). Figure 1A shows a sample of SSRs recorded in a young woman (patient 3).
Neurophysiological evidence of a small-fibers neuropathy (SFN). A Sympathetic skin responses (SSRs) in a representative patient. Responses were recorded from the lower and the upper limbs at the right side. Note that both responses were not recordable. Responses were obtained by stimulating the wrist with the intensity set at 20 mA. B Histogram showing significant differences in terms of SSR latencies between patients and controls (data are reported as mean values ± S.D. ** p < 0.01)
In remaining cases, SSR latencies did not change between patients and controls when recorded from the upper limbs (1.59 ± 0.25 s versus 1.44 ± 0.33 s: p = 0.27), whereas they are significantly longer compared to healthy volunteers when recorded from feet (1.98 ± 0.27 s versus 1.56 ± 0.29 s: p = 0.0019, unpaired t-test with Welch’s correction; Fig. 1B).
Quantitative electromyography (MMUA)
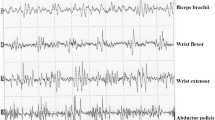
MMUA parameters did not significantly differ between healthy controls and patients (Table 2); in particular, both in deltoid and TA muscle, the percentage of polyphasia (D: p = 0.24; TA: p = 0.35), MUP area (p = 0.45; p = 0.44), amplitude (p = 0.27; p = 0.63), and mean duration (p = 0.06; p = 0.45) did not change between the two groups. Figure 2 shows MUPs recorded from both deltoid and TA muscle in a representative patient (female, patient 3).
Quantitative EMG in a representative long-COVID patient. A Multi-motor unit analysis (MMUA) was performed on tibialis anterior (TA) muscle, on the right side, collecting 22 different MUPs. A Cartesian graph on the left (bottom) shows the distribution of each MUP in terms of amplitude (y-axis, expressed as mV) and duration (x-axis, ms). B MMUA was performed on deltoid muscle, on the left side, collecting 23 different MUPs. A Cartesian graph on the left (bottom) shows the distribution of each MUP in terms of amplitude (y-axis, expressed as mV) and duration (x-axis, ms). Note that almost all MUPs are within the normal range, and traces show MUPs with a typical triphasic appearance in both muscles
Discussion
Our data suggest a predominant involvement of the autonomic system, with a focus on cholinergic efferent sympathetic activity, in patients with pain and “long-COVID syndrome,” without any evidence of myopathic changes. There is no correlation between disease severity and electrophysiological findings. To the best of our knowledge, this is the first report combining a neurophysiological evaluation of small fibers with quantitative electromyography (qEMG). Our findings could also contribute to understand pathogenetic mechanisms underlying SARS-CoV-2 neurotropism, possibly guiding both pharmacological and non-pharmacological treatments for long-COVID syndrome. For example, in absence of a clear etiopathogenesis, rehabilitative protocols currently refer to treatments for pathologies with similar clinical presentation, i.e., fibromyalgia or chronic fatigue syndrome, which have a different pathogenesis and underpinning mechanisms [11].
Our results are in line with other studies pointing to a predominant involvement of small fibers in long-COVID [21, 22] and fit with neurophysiological and histopathological data coming from hospitalized patients [1, 23]. Oaklander and colleagues described skin biopsies in long-COVID and confirmed a predominant neuropathic involvement [21]; the hypothesis of an underlying small-fiber pathology is also corroborated by the observation of small-fiber loss applying in vivo corneal confocal microscopy [24]. Also, Abrams and co-workers found changes in skin biopsies supporting the hypothesis of a small fiber neuropathy (SFN) [25].
In a more recent study, SSRs have been assessed in 70 patients, who recovered from COVID-19, showing significantly longer latencies in patients when compared to the control group; although the temporal relationship with the primary infection has not been reported in detail, the authors concluded that SFN can predict disease severity, as well as clinical outcomes and prognosis [26].
Nonetheless, one study reported predominant myopathic features in long-COVID patients, using a neurophysiological protocol similar to that we applied here [8]. The authors based their conclusions on MUP duration and polyphasia found in tibialis anterior (TA) muscle; no changes in terms of MUP amplitude, duration, number of turns, and polyphasia were additionally found in deltoid muscle. Moreover, authors did not perform muscle biopsies, although another study has recently corroborated these findings by skeletal muscle histopathology [27]. Differences between these results and ours may be additionally related to other factors: In some cases, their patients had been hospitalized during the acute phase of the illness, and they were older than ours at the time of first electrophysiological evaluation. Moreover, time elapsed from the infection, and the electrophysiological assessment was longer in our sample. Finally, Agergaard and co-workers did not clarify whether and which myotoxic drugs had been administered during the acute phase, including chloroquine, steroids, ritonavir, or lopinavir.
Overall, it is worth considering that different neurophysiological observations may be explained, at least in part, by different viral variants, possibly triggering different acute and chronic neurological manifestations.
In our sample, patients showing an autonomic involvement were treated with steroids (prednisone; starting dose, 25 mg/day), with a subjective improvement of both sensory and muscle symptoms.
Although our neurophysiological data are strongly corroborated by histopathological findings from other laboratories, they have some limitations. First, we did not perform a skin biopsy in our patients. Second, there is no follow-up of our neurophysiological findings, except for one patient. Third, autonomic changes were assessed by evaluating SSRs only; we did not evaluate the patients for the presence of autonomic symptoms, such as impaired sweating or fluctuations in blood pressure and the heart rate. Equally important, laser-evoked potentials (LEPs) were not assessed. LEPs represent the most sensitive test for the diagnosis of SFN; they allow to evaluate both A-delta and C fibers, highlighting at the same time possible changes at a cortical level, including primary sensory and cingulate cortices [28]. Finally, fatigue and pain should not only be attributed to involvement of the small fibers; they could also be related to central nervous system (CNS) involvement, cardiac involvement, kidney failure, lung disease or anxiety, and depression [29, 30].
Data availability
The corresponding author has full access to data and has the right to publish such data. Data will be available upon reasonable request to the corresponding author.
Code availability
Not applicable.
References
Bulfamante G, Bocci T, Falleni M et al (2021) Brainstem neuropathology in two cases of COVID-19: SARS-CoV-2 trafficking between brain and lung. J Neurol 268:4486–4491. https://doi.org/10.1007/s00415-021-10604-8
Matschke J, Lütgehetmann M, Hagel C et al (2020) Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol 19:919–929. https://doi.org/10.1016/S1474-4422(20)30308-2
Krasemann S, Glatzel M, Pless O (2022) Response to: SARS-CoV-2 and type I interferon signaling in brain endothelial cells: blurring the lines between friend or foe. Stem Cell Reports 17:1014–1015. https://doi.org/10.1016/j.stemcr.2022.04.012
Schwabenland M, Salié H, Tanevski J et al (2021) Deep spatial profiling of human COVID-19 brains reveals neuroinflammation with distinct microanatomical microglia-T-cell interactions. Immunity 54:1594-1610.e11. https://doi.org/10.1016/j.immuni.2021.06.002
Ferrucci R, Dini M, Rosci C et al (2022) One-year cognitive follow-up of COVID-19 hospitalized patients. Eur J Neurol 29:2006–2014. https://doi.org/10.1111/ene.15324
Sykes DL, Holdsworth L, Jawad N et al (2021) Post-COVID-19 symptom burden: what is long-COVID and how should we manage it? Lung 199:113–119. https://doi.org/10.1007/s00408-021-00423-z
Versace V, Ortelli P, Dezi S et al (2023) Co-ultramicronized palmitoylethanolamide/luteolin normalizes GABAB-ergic activity and cortical plasticity in long COVID-19 syndrome. Clin Neurophysiol 145:81–88. https://doi.org/10.1016/j.clinph.2022.10.017
Agergaard J, Leth S, Pedersen TH et al (2021) Myopathic changes in patients with long-term fatigue after COVID-19. Clin Neurophysiol 132:1974–1981. https://doi.org/10.1016/j.clinph.2021.04.009
Hejbøl EK, Harbo T, Agergaard J et al (2022) Reply to “Post-COVID myopathy.” European J Neurol 29:3752–3753. https://doi.org/10.1111/ene.15525
Villa D, Ardolino G, Borellini L et al (2021) Subclinical myopathic changes in COVID-19. Neurol Sci 42:3973–3979. https://doi.org/10.1007/s10072-021-05469-8
Khoja O, Silva Passadouro B, Mulvey M et al (2022) Clinical characteristics and mechanisms of musculoskeletal pain in long COVID. J Pain Res 15:1729–1748. https://doi.org/10.2147/JPR.S365026
Elie B, Guiheneuc P (1990) Sympathetic skin response: normal results in different experimental conditions. Electroencephalogr Clin Neurophysiol 76:258–267. https://doi.org/10.1016/0013-4694(90)90020-k
Nasri A, Kacem I, Farhat N et al (2022) Heart rate variability and sympathetic skin response for the assessment of autonomic dysfunction in leucine-rich repeat kinase 2 associated Parkinson’s disease. Neurophysiol Clin 52:81–93. https://doi.org/10.1016/j.neucli.2021.12.007
Tankisi H, Pugdahl K, Johnsen B, Fuglsang-Frederiksen A (2007) Correlations of nerve conduction measures in axonal and demyelinating polyneuropathies. Clin Neurophysiol 118:2383–2392. https://doi.org/10.1016/j.clinph.2007.07.027
Nandedkar SD, Barkhaus PE, Charles A (1995) Multi-motor unit action potential analysis (MMA). Muscle Nerve 18:1155–1166. https://doi.org/10.1002/mus.880181012
Stålberg E, Falck B, Sonoo M et al (1995) Multi-MUP EMG analysis–a two year experience in daily clinical work. Electroencephalogr Clin Neurophysiol 97:145–154. https://doi.org/10.1016/0924-980x(95)00007-8
Stålberg E, van Dijk H, Falck B et al (2019) Standards for quantification of EMG and neurography. Clin Neurophysiol 130:1688–1729. https://doi.org/10.1016/j.clinph.2019.05.008
Podnar S, Vodusek DB, Stålberg E (2002) Comparison of quantitative techniques in anal sphincter electromyography. Muscle Nerve 25:83–92. https://doi.org/10.1002/mus.10017
Stålberg E, van Dijk H, Falck B et al (2019) Standards for quantification of EMG and neurography. Clin Neurophysiol 130(9):1688–1729. https://doi.org/10.1016/j.clinph.2019.05.008
Abraham A, Alabdali M, Alsulaiman A et al (2017) Repetitive nerve stimulation cutoff values for the diagnosis of myasthenia gravis. Muscle Nerve 55(2):166–170. https://doi.org/10.1002/mus.25214
Oaklander AL, Mills AJ, Kelley M et al (2022) Peripheral neuropathy evaluations of patients with prolonged long COVID. Neurol Neuroimmunol Neuroinflamm 9:e1146. https://doi.org/10.1212/NXI.0000000000001146
Safavi F, Gustafson L, Walitt B et al (2022) Neuropathic symptoms with SARS-CoV-2 vaccination. MedRxiv 12:22274439. https://doi.org/10.1101/2022.05.16.22274439
Bocci T, Bulfamante G, Campiglio L et al (2021) Brainstem clinical and neurophysiological involvement in COVID-19. J Neurol 268:3598–3600. https://doi.org/10.1007/s00415-021-10474-0
Bitirgen G, Korkmaz C, Zamani A et al (2022) Corneal confocal microscopy identifies corneal nerve fibre loss and increased dendritic cells in patients with long COVID. Br J Ophthalmol 106:1635–1641. https://doi.org/10.1136/bjophthalmol-2021-319450
Abrams RMC, Simpson DM, Navis A, Jette N, Zhou L, Shin SC (2022) Small fiber neuropathy associated with SARS-CoV-2 infection. Muscle Nerve 65(4):440–443. https://doi.org/10.1002/mus.27458
Roshanzamir S, Mohamadi Jahromi LS (2022) Study of sympathetic skin response in patients with COVID-19 infection. Acta Neurol Belg 1–7. https://doi.org/10.1007/s13760-022-02120-x
Hejbøl EK, Harbo T, Agergaard J et al (2022) Myopathy as a cause of fatigue in long-term post-COVID-19 symptoms: evidence of skeletal muscle histopathology. Eur J Neurol 29:2832–2841. https://doi.org/10.1111/ene.15435
Lefaucheur JP, Wahab A, Planté-Bordeneuve V et al (2015) Diagnosis of small fiber neuropathy: a comparative study of five neurophysiological tests. Neurophysiol Clin 45(6):445–455. https://doi.org/10.1016/j.neucli.2015.09.012
Campos MC, Nery T, Starke AC et al (2022) Post-viral fatigue in COVID-19: a review of symptom assessment methods, mental, cognitive, and physical impairment. Neurosci Biobehav Rev 142:104902. https://doi.org/10.1016/j.neubiorev.2022.104902
Mantovani E, Mariotto S, Gabbiani D et al (2021) Chronic fatigue syndrome: an emerging sequela in COVID-19 survivors? J Neurovirol 27(4):631–637. https://doi.org/10.1007/s13365-021-01002-x
Acknowledgements
The authors are grateful to Mr. Denis Dreon for his excellent technical assistance, as well as patients and healthy volunteers for their participation.
Funding
Open access funding provided by Università degli Studi di Milano within the CRUI-CARE Agreement. This study was supported by the donation of the “Romeo and Enrica Invernizzi” Foundation (“Bando COVID-19”).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
We confirm that we have read the journal’s position on issues involved in ethical publication and that this report is consistent with those guidelines.
Consent to participate
The study was approved by the institutional review board and the ethics committee at “Azienda Socio-Sanitaria Territoriale Santi Paolo e Carlo, Milano, Italia.” The study has been conducted in accordance with the ethical standards laid down in the Declaration of Helsinki.
Conflict of interest
The authors declare no competing interests.
Informed consent
The study was approved by the institutional review board and the ethics committee at “Azienda Socio-Sanitaria Territoriale Santi Paolo e Carlo, Milano, Italia”. The study has been conducted in accordance with the ethical standards laid down in the Declaration of Helsinki.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bocci, T., Bertini, A., Campiglio, L. et al. Not myopathic, but autonomic changes in patients with long-COVID syndrome: a case series. Neurol Sci 44, 1147–1153 (2023). https://doi.org/10.1007/s10072-023-06637-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-023-06637-8