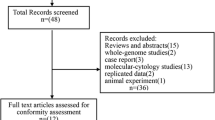
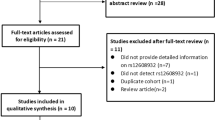
Abstract
Recently, NEK1 (NIMA-related kinase 1) mutations were identified as a cause of amyotrophic lateral sclerosis (ALS), but the relationship between them remains unclear owing to the small sample size and low mutation rate. We made a meta-analysis to make clear the relationship. Eight case-control studies involving 8603 cases and 18,695 controls were enrolled. Results demonstrated that the frequency of NEK1 mutations was 3.1% (95% CI 2.5–3.8%) in ALS patients, including the frequencies of loss of function (LoF) and missense mutations, which were 0.9% (95% CI 0.6–1.1%) and 2.3% (95% CI 1.7–2.8%) in ALS patients, respectively. NEK1 mutations (OR 2.14; 95% CI 1.81–2.52; p < 0.001), including LoF mutations (OR 6.93; 95% CI 4.38–10.96; p < 0.001) and missense mutations (OR 1.65; 95% CI 1.37–1.99; p < 0.001) were associated with a significantly increased risk for ALS. And the risk of NEK1 LoF mutations (OR 6.93) is more than four times of that of NEK1 missense mutations (OR 1.65). Subgroup analysis suggested that the frequency of LoF mutations was higher in European patients (1%) than that in Asian patients (0.7%). In conclusion, NEK1 LoF and missense mutations are low frequencies in ALS patients, but both of them are associated with the increased risk for ALS. Altogether, NEK1 mutations including LoF mutations and missense mutations are more associated with Asian patients than European patients.








Similar content being viewed by others
References
Siddique N, Siddique T (1993-2020) Amyotrophic Lateral Sclerosis Overview. Adam MP, Ardinger HH, Pagon RA, et al., (eds). GeneReviews® [Internet]. University of Washington, Seattle
Taylor JP, Brown RH Jr, Cleveland DW (2016) Decoding ALS: from genes to mechanism. Nature 539(7628):197–206
Turner MR et al (2013) Controversies and priorities in amyotrophic lateral sclerosis. The Lancet. Neurology 12(3):310–322
Rosen DR (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 364(6435):362
Vance C et al (2009) Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science 323(5918):1208–1211
Sreedharan J et al (2008) TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 319(5870):1668–1672
Teyssou E et al (2013) Mutations in SQSTM1 encoding p62 in amyotrophic lateral sclerosis: genetics and neuropathology. Acta Neuropathol 125(4):511–522
Beers DR, Appel SH (2019) Immune dysregulation in amyotrophic lateral sclerosis: mechanisms and emerging therapies. Lancet Neurol 18(2):211–220
van Es MA et al (2017) Amyotrophic lateral sclerosis. Lancet 390(10107):2084–2098
Feige E et al (2006) Nek1 shares structural and functional similarities with NIMA kinase. Biochim Biophys Acta 1763(3):272–281
Fry AM et al (2012) Cell cycle regulation by the NEK family of protein kinases. J Cell Sci 125(Pt 19):4423–4433
Amin P et al (2018) Regulation of a distinct activated RIPK1 intermediate bridging complex I and complex II in TNFalpha-mediated apoptosis. Proc Natl Acad Sci USA 115(26):E5944–e5953
Chen Y et al (2008) Never-in-mitosis related kinase 1 functions in DNA damage response and checkpoint control. Cell Cycle 7(20):3194–3201
Higelin J et al (2018) NEK1 loss-of-function mutation induces DNA damage accumulation in ALS patient-derived motoneurons. Stem Cell Res 30:150–162
Shalom O et al (2008) The mammalian Nek1 kinase is involved in primary cilium formation. FEBS Lett 582(10):1465–1470
White MC, Quarmby LM (2008) The NIMA-family kinase, Nek1 affects the stability of centrosomes and ciliogenesis. BMC Cell Biol 9:29
Cirulli ET et al (2015) Exome sequencing in amyotrophic lateral sclerosis identifies risk genes and pathways. Science 347(6229):1436–1441
Edens BM, Miller N, Ma YC (2016) Impaired Autophagy and Defective Mitochondrial Function: Converging Paths on the Road to Motor Neuron Degeneration. Front Cell Neurosci 10:44
Kenna KP et al (2016) NEK1 variants confer susceptibility to amyotrophic lateral sclerosis. Nat Genet 48(9):1037–1042
Gratten J et al (2017) Whole-exome sequencing in amyotrophic lateral sclerosis suggests NEK1 is a risk gene in Chinese. Genome Med 9(1):97
Brenner D et al (2016) NEK1 mutations in familial amyotrophic lateral sclerosis. Brain 139:CP14–CP17
Black HA et al (2017) Genetic epidemiology of motor neuron disease-associated variants in the Scottish population. Neurobiol Aging 51:178.e11–178.e20
Nguyen HP et al (2018) NEK1 genetic variability in a Belgian cohort of ALS and ALS-FTD patients. Neurobiol Aging 61:255.e1–255.e7
Shu S et al (2018) Mutation screening of NEK1 in Chinese ALS patients. Neurobiol Aging 71:267.e1–267.e4
Stang A (2010) Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25(9):603–605
Naruse H, Ishiura H, Mitsui J et al (2020) Loss-of-function variants in NEK1 are associated with an increased risk of sporadic ALS in the Japanese population. J Hum Genet. https://doi.org/10.1038/s10038-020-00830-9
Acknowledgments
The authors thank all those who collaborated in the analysis, interpretation of data, and writing the article. This work was supported by the Natural Science Foundation of Shandong Province under Grant [number ZR2014HM064].
Funding
Natural Science Foundation of Shandong Province under Grant.[number ZR2014HM064].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
None.
Informed consent
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Rights and permissions
About this article
Cite this article
Yao, L., He, X., Cui, B. et al. NEK1 mutations and the risk of amyotrophic lateral sclerosis (ALS): a meta-analysis. Neurol Sci 42, 1277–1285 (2021). https://doi.org/10.1007/s10072-020-05037-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-020-05037-6