Abstract
Introduction
Parkinson’s disease (PD) is a motor disorder that initially presents with unilateral symptoms. Widespread white matter (WM) alterations have been reported since the early stages of the disease. The aim of this study was to investigate WM alterations in right-dominant and left-dominant symptom PD patients (RPD and LPD, respectively) with respect to healthy controls (HC) by diffusion-weighted magnetic resonance imaging (MRI).
Methods
Thirty-eight subjects participated in this study: 12 RPD (median H&Y [IQR] = 1.5 [1.1–2], median UPDRS III [IQR] = 23 [7.8–25]), 9 LPD (median H&Y [IQR] = 1.5 [1–2.5], median UPDRS III [IQR] = 17 [12–22]), and 17 HC. All the participants were scanned on a 1.5-T MRI scanner. Maps of fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) were computed for all the subjects. Tract-based spatial statistics (TBSS) was performed for each diffusion parameter, to test WM differences between RPD, LPD, and HC (ANCOVA design). Family-wise error (FWE) correction was performed and p values lower than 0.05 were considered significant.
Results
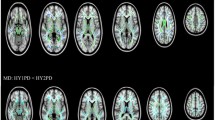
No significant FA and RD differences were observed between RPD, LPD, and HC. Significantly increased MD and AD were observed in RPD with respect to HC within widespread WM regions, bilaterally. Conversely, no significant WM alterations were detected in LPD.
Conclusion
WM integrity was found to be significantly altered in RPD but not in LPD, suggesting that LPD profile may be associated to more favorable prognosis. Since clinical laterality onset may affect the extent of WM integrity changes, it should be taken into account in neuroimaging studies investigating PD.


Similar content being viewed by others
References
De Pablo-Fernandez E, Lees AJ, Holton JL, Warner TT (2019) Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2018.4377
Ham JH, Lee JJ, Kim JS, Lee PH, Sohn YH (2015) Is dominant-side onset associated with a better motor compensation in Parkinson’s disease? Mov Disord 30(14):1921–1925. https://doi.org/10.1002/mds.26418
Papagno C, Trojano L (2018) Cognitive and behavioral disorders in Parkinson’s disease: an update. I: cognitive impairments. Neurol Sci 39(2):215–223. https://doi.org/10.1007/s10072-017-3154-8
Trojano L, Papagno C (2018) Cognitive and behavioral disorders in Parkinson’s disease: an update. II: behavioral disorders. Neurol Sci 39(1):53–61. https://doi.org/10.1007/s10072-017-3155-7
Heinrichs-Graham E, Santamaria PM, Gendelman HE, Wilson TW (2017) The cortical signature of symptom laterality in Parkinson’s disease. NeuroImage Clin 14:433–440. https://doi.org/10.1016/j.nicl.2017.02.010
Frazzitta G, Ferrazzoli D, Maestri R, Rovescala R, Guaglio G, Bera R, Volpe D, Pezzoli G (2015) Differences in muscle strength in parkinsonian patients affected on the right and left side. PLoS One 10(3):e0121251. https://doi.org/10.1371/journal.pone.0121251
Baumann CR, Held U, Valko PO, Wienecke M, Waldvogel D (2014) Body side and predominant motor features at the onset of Parkinson’s disease are linked to motor and nonmotor progression. Mov Disord 29(2):207–213. https://doi.org/10.1002/mds.25650
Munhoz RP, Espay AJ, Morgante F, Li JY, Teive HA, Dunn E, Gallin E, Litvan I (2013) Long-duration Parkinson’s disease: role of lateralization of motor features. Parkinsonism Relat Disord 19(1):77–80. https://doi.org/10.1016/j.parkreldis.2012.07.008
Scherfler C, Seppi K, Mair KJ, Donnemiller E, Virgolini I, Wenning GK, Poewe W (2012) Left hemispheric predominance of nigrostriatal dysfunction in Parkinson’s disease. Brain 135(Pt 11):3348–3354. https://doi.org/10.1093/brain/aws253
Minkova L, Habich A, Peter J, Kaller CP, Eickhoff SB, Kloppel S (2017) Gray matter asymmetries in aging and neurodegeneration: a review and meta-analysis. Hum Brain Mapp 38(12):5890–5904. https://doi.org/10.1002/hbm.23772
Di Tella S, Baglio F, Cabinio M, Nemni R, Traficante D, Silveri MC (2018) Selection processing in noun and verb production in left- and right-sided Parkinson’s disease patients. Front Psychol 9:1241. https://doi.org/10.3389/fpsyg.2018.01241
Kim JS, Yang JJ, Lee JM, Youn J, Kim JM, Cho JW (2014) Topographic pattern of cortical thinning with consideration of motor laterality in Parkinson disease. Parkinsonism Relat Disord 20(11):1186–1190. https://doi.org/10.1016/j.parkreldis.2014.08.021
Agosta F, Galantucci S, Filippi M (2017) Advanced magnetic resonance imaging of neurodegenerative diseases. Neurol Sci 38(1):41–51. https://doi.org/10.1007/s10072-016-2764-x
Rektor I, Svatkova A, Vojtisek L, Zikmundova I, Vanicek J, Kiraly A, Szabo N (2018) White matter alterations in Parkinson’s disease with normal cognition precede grey matter atrophy. PLoS One 13(1):e0187939. https://doi.org/10.1371/journal.pone.0187939
Pozorski V, Oh JM, Adluru N, Merluzzi AP, Theisen F, Okonkwo O, Barzgari A, Krislov S, Sojkova J, Bendlin BB, Johnson SC, Alexander AL, Gallagher CL (2018) Longitudinal white matter microstructural change in Parkinson’s disease. Hum Brain Mapp 39(10):4150–4161. https://doi.org/10.1002/hbm.24239
Winklewski PJ, Sabisz A, Naumczyk P, Jodzio K, Szurowska E, Szarmach A (2018) Understanding the physiopathology behind axial and radial diffusivity changes-what do we know? Front Neurol 9:92. https://doi.org/10.3389/fneur.2018.00092
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30(12):1591–1601. https://doi.org/10.1002/mds.26424
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25(15):2649–2653. https://doi.org/10.1002/mds.23429
Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, Giladi N, Holloway RG, Moore CG, Wenning GK, Yahr MD, Seidl L, Movement Disorder Society Task Force on Rating Scales for Parkinson’s D (2004) Movement Disorder Society task force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord 19(9):1020–1028. https://doi.org/10.1002/mds.20213
Santangelo G, Siciliano M, Pedone R, Vitale C, Falco F, Bisogno R, Siano P, Barone P, Grossi D, Santangelo F, Trojano L (2015) Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol Sci 36(4):585–591. https://doi.org/10.1007/s10072-014-1995-y
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23(Suppl 1):S208–S219. https://doi.org/10.1016/j.neuroimage.2004.07.051
Andersson JL, Skare S, Ashburner J (2003) How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage 20(2):870–888. https://doi.org/10.1016/S1053-8119(03)00336-7
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17(3):143–155. https://doi.org/10.1002/hbm.10062
Andersson JLR, Sotiropoulos SN (2016) An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage 125:1063–1078. https://doi.org/10.1016/j.neuroimage.2015.10.019
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 31(4):1487–1505. https://doi.org/10.1016/j.neuroimage.2006.02.024
Andersson JL, Jenkinson M, Smith S (2007) Non-linear registration, aka spatial normalisation. FMRIB technical report TR07JA2. FMRIB Analysis Group of the University of Oxford
Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014) Permutation inference for the general linear model. NeuroImage 92:381–397. https://doi.org/10.1016/j.neuroimage.2014.01.060
Mori S, Wakana S, Nagae-Poetscher LM, Van Zijl PCM (2006) MRI atlas of human white matter. American Journal of Neuroradiology, 27(6):1384
Bohnen NI, Albin RL, Koeppe RA, Wernette KA, Kilbourn MR, Minoshima S, Frey KA (2006) Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab 26(9):1198–1212. https://doi.org/10.1038/sj.jcbfm.9600276
Zhang Y, Wu IW, Buckley S, Coffey CS, Foster E, Mendick S, Seibyl J, Schuff N (2015) Diffusion tensor imaging of the nigrostriatal fibers in Parkinson’s disease. Mov Disord 30(9):1229–1236. https://doi.org/10.1002/mds.26251
Atkinson-Clement C, Pinto S, Eusebio A, Coulon O (2017) Diffusion tensor imaging in Parkinson’s disease: review and meta-analysis. NeuroImage Clin 16:98–110. https://doi.org/10.1016/j.nicl.2017.07.011
Fling BW, Curtze C, Horak FB (2018) Gait asymmetry in people with Parkinson’s disease is linked to reduced integrity of callosal sensorimotor regions. Front Neurol 9:215. https://doi.org/10.3389/fneur.2018.00215
Acknowledgments
Sonia Di Tella received a scholarship from Crespi Spano Foundation.
Funding
This study was in part funded by the Italian Ministry of Health (Ricerca Corrente 2016–2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the IRCSS Fondazione Don Carlo Gnocchi Ethics Committee and it was performed in accordance with the principles of the Helsinki Declaration. All the participants provided their written and informed consent.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pelizzari, L., Di Tella, S., Laganà, M.M. et al. White matter alterations in early Parkinson’s disease: role of motor symptom lateralization. Neurol Sci 41, 357–364 (2020). https://doi.org/10.1007/s10072-019-04084-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-019-04084-y