Abstract
Magnetic resonance imaging (MRI) is playing an increasingly important role in the study of neurodegenerative diseases, delineating the structural and functional alterations determined by these conditions. Advanced MRI techniques are of special interest for their potential to characterize the signature of each neurodegenerative condition and aid both the diagnostic process and the monitoring of disease progression. This aspect will become crucial when disease-modifying (personalized) therapies will be established. MRI techniques are very diverse and go from the visual inspection of MRI scans to more complex approaches, such as manual and automatic volume measurements, diffusion tensor MRI, and functional MRI. All these techniques allow us to investigate the different features of neurodegeneration. In this review, we summarize the most recent advances concerning the use of MRI in some of the most important neurodegenerative conditions, putting an emphasis on the advanced techniques.

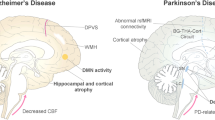
Reproduced from [12] with permission

Reproduced from [33] with permission

Reproduced from [52] with permission

Reproduced from [75] with permission
Similar content being viewed by others
References
Agosta F, Weiler M, Filippi M (2015) Propagation of pathology through brain networks in neurodegenerative diseases: from molecules to clinical phenotypes. CNS Neurosci Ther 21:754–767
Teipel S, Drzezga A, Grothe MJ et al (2015) Multimodal imaging in Alzheimer’s disease: validity and usefulness for early detection. Lancet Neurol 14:1037–1053
Gosche KM, Mortimer JA, Smith CD et al (2002) Hippocampal volume as an index of Alzheimer neuropathology: findings from the Nun Study. Neurology 58:1476–1482
Thompson PM, Hayashi KM, de Zubicaray G et al (2003) Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci 23:994–1005
Ferreira LK, Diniz BS, Forlenza OV et al (2011) Neurostructural predictors of Alzheimer’s disease: a meta-analysis of VBM studies. Neurobiol Aging 32:1733–1741
Albert MS, DeKosky ST, Dickson D et al (2011) The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:270–279
Dubois B, Feldman HH, Jacova C et al (2010) Revising the definition of Alzheimer’s disease: a new lexicon. Lancet Neurol 9:1118–1127
McKhann GM, Knopman DS, Chertkow H et al (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7:263–269
Thompson PM, Mega MS, Woods RP et al (2001) Cortical change in Alzheimer’s disease detected with a disease-specific population-based brain atlas. Cereb Cortex 11:1–16
Perrotin A, de Flores R, Lamberton F et al (2015) Hippocampal subfield volumetry and 3D surface mapping in subjective cognitive decline. J Alzheimers Dis 48(Suppl 1):S141–S150
Khan W, Westman E, Jones N et al (2015) Automated hippocampal subfield measures as predictors of conversion from mild cognitive impairment to Alzheimer’s disease in two independent cohorts. Brain Topogr 28:746–759
Kerchner GA, Deutsch GK, Zeineh M et al (2012) Hippocampal CA1 apical neuropil atrophy and memory performance in Alzheimer’s disease. Neuroimage 63:194–202
Sexton CE, Kalu UG, Filippini N et al (2011) A meta-analysis of diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 32:2322-e5–2322-e18
Canu E, Frisoni GB, Agosta F et al (2013) Early and late onset Alzheimer’s disease patients have distinct patterns of white matter damage. Neurobiol Aging 33:1023–1033
Zhuang L, Sachdev PS, Trollor JN et al (2012) Microstructural white matter changes in cognitively normal individuals at risk of amnestic MCI. Neurology 79:748–754
Clerx L, Visser PJ, Verhey F, Aalten P (2012) New MRI markers for Alzheimer’s disease: a meta-analysis of diffusion tensor imaging and a comparison with medial temporal lobe measurements. J Alzheimers Dis 29:405–429
Scola E, Bozzali M, Agosta F et al (2010) A diffusion tensor MRI study of patients with MCI and AD with a 2-year clinical follow-up. J Neurol Neurosurg Psychiatry 81:798–805
Douaud G, Menke RA, Gass A et al (2013) Brain microstructure reveals early abnormalities more than 2 years prior to clinical progression from mild cognitive impairment to Alzheimer’s disease. J Neurosci 33:2147–2155
Dyrba M, Barkhof F, Fellgiebel A et al (2015) Predicting prodromal Alzheimer’s disease in subjects with mild cognitive impairment using machine learning classification of multimodal multicenter diffusion-tensor and magnetic resonance imaging data. J Neuroimaging 25:738–747
Greicius MD, Srivastava G, Reiss AL, Menon V (2004) Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA 101:4637–4642
Agosta F, Pievani M, Geroldi C et al (2012) Resting state fMRI in Alzheimer’s disease: beyond the default mode network. Neurobiol Aging 33:1564–1578
Koch W, Teipel S, Mueller S et al (2012) Diagnostic power of default mode network resting state fMRI in the detection of Alzheimer’s disease. Neurobiol Aging 33:466–478
Bai F, Liao W, Watson DR et al (2011) Abnormal whole-brain functional connection in amnestic mild cognitive impairment patients. Behav Brain Res 216:666–672
Petrella JR, Sheldon FC, Prince SE et al (2011) Default mode network connectivity in stable vs progressive mild cognitive impairment. Neurology 76:511–517
Burton EJ, Barber R, Mukaetova-Ladinska EB et al (2009) Medial temporal lobe atrophy on MRI differentiates Alzheimer’s disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain 132:195–203
Firbank MJ, Blamire AM, Teodorczuk A et al (2010) High resolution imaging of the medial temporal lobe in Alzheimer’s disease and dementia with Lewy bodies. J Alzheimers Dis 21:1129–1140
Vemuri P, Simon G, Kantarci K et al (2011) Antemortem differential diagnosis of dementia pathology using structural MRI: differential-STAND. Neuroimage 55:522–531
Burton EJ, Karas G, Paling SM et al (2002) Patterns of cerebral atrophy in dementia with Lewy bodies using voxel based morphometry. Neuroimage 17:618–630
Bozzali M, Falini A, Cercignani M et al (2005) Brain tissue damage in dementia with Lewy bodies: an in vivo diffusion tensor MRI study. Brain 128:1595–1604
Kantarci K, Avula R, Senjem ML et al (2010) Dementia with Lewy bodies and Alzheimer disease: neurodegenerative patterns characterized by DTI. Neurology 74:1814–1821
Delli Pizzi S, Franciotti R, Taylor JP et al (2015) Structural connectivity is differently altered in dementia with Lewy body and Alzheimer’s disease. Front Aging Neurosci 7:208
Watson R, Blamire AM, Colloby SJ et al (2012) Characterizing dementia with Lewy bodies by means of diffusion tensor imaging. Neurology 79:906–914
Firbank MJ, Watson R, Mak E et al (2016) Longitudinal diffusion tensor imaging in dementia with Lewy bodies and Alzheimer’s disease. Parkinsonism Relat Disord 24:76–80
Galvin JE, Price JL, Yan Z et al (2011) Resting BOLD fMRI differentiates dementia with Lewy bodies vs Alzheimer disease. Neurology 76:1797–1803
Lowther ER, O’Brien JT, Firbank MJ, Blamire AM (2014) Lewy body compared with Alzheimer dementia is associated with decreased functional connectivity in resting state networks. Psychiatry Res 223:192–201
Kenny ER, Blamire AM, Firbank MJ, O’Brien JT (2012) Functional connectivity in cortical regions in dementia with Lewy bodies and Alzheimer’s disease. Brain 135:569–581
Peraza LR, Kaiser M, Firbank M et al (2014) fMRI resting state networks and their association with cognitive fluctuations in dementia with Lewy bodies. Neuroimage Clin 4:558–565
Seeley WW, Crawford R, Rascovsky K et al (2008) Frontal paralimbic network atrophy in very mild behavioral variant frontotemporal dementia. Arch Neurol 65:249–255
Du AT, Schuff N, Kramer JH et al (2007) Different regional patterns of cortical thinning in Alzheimer’s disease and frontotemporal dementia. Brain 130:1159–1166
Rohrer JD, Lashley T, Schott JM et al (2011) Clinical and neuroanatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain 134:2565–2581
Agosta F, Scola E, Canu E et al (2012) White matter damage in frontotemporal lobar degeneration spectrum. Cereb Cortex 22:2705–2714
Whitwell JL, Avula R, Senjem ML et al (2011) Gray and white matter water diffusion in the syndromic variants of frontotemporal dementia. Neurology 74:1279–1287
Mahoney CJ, Ridgway GR, Malone IB et al (2014) Profiles of white matter tract pathology in frontotemporal dementia. Hum Brain Mapp 35:4163–4179
Farb NA, Grady CL, Strother S et al (2013) Abnormal network connectivity in frontotemporal dementia: evidence for prefrontal isolation. Cortex 49:1856–1873
Filippi M, Agosta F, Scola E et al (2013) Functional network connectivity in the behavioral variant of frontotemporal dementia. Cortex 49:2389–2401
Zhou J, Greicius MD, Gennatas ED et al (2010) Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133:1352–1367
Agosta F, Sala S, Valsasina P et al (2013) Brain network connectivity assessed using graph theory in frontotemporal dementia. Neurology 81:134–143
Gorno-Tempini ML, Dronkers NF, Rankin KP et al (2004) Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol 55:335–346
Rohrer JD, Warren JD, Modat M et al (2009) Patterns of cortical thinning in the language variants of frontotemporal lobar degeneration. Neurology 72:1562–1569
van de Pol LA, Hensel A, van der Flier WM et al (2006) Hippocampal atrophy on MRI in frontotemporal lobar degeneration and Alzheimer’s disease. J Neurol Neurosurg Psychiatry 77:439–442
Agosta F, Henry RG, Migliaccio R et al (2010) Language networks in semantic dementia. Brain 133:286–299
Galantucci S, Tartaglia MC, Wilson SM et al (2011) White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain 134:3011–3029
Caverzasi E, Henry RG, Vitali P et al (2014) Application of quantitative DTI metrics in sporadic CJD. Neuroimage Clin 4:426–435
Catani M, Mesulam MM, Jakobsen E et al (2013) A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain 136:2619–2628
Guo CC, Gorno-Tempini ML, Gesierich B et al (2013) Anterior temporal lobe degeneration produces widespread network-driven dysfunction. Brain 136:2979–2991
Agosta F, Galantucci S, Valsasina P et al (2014) Disrupted brain connectome in semantic variant of primary progressive aphasia. Neurobiol Aging 35:2646–2655
Peran P, Cherubini A, Assogna F et al (2010) Magnetic resonance imaging markers of Parkinson’s disease nigrostriatal signature. Brain 133:3423–3433
Du G, Lewis MM, Styner M et al (2011) Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson’s disease. Mov Disord 26:1627–1632
Menke RA, Scholz J, Miller KL et al (2009) MRI characteristics of the substantia nigra in Parkinson’s disease: a combined quantitative T1 and DTI study. Neuroimage 47:435–441
Kwon DH, Kim JM, Oh SH et al (2012) Seven-Tesla magnetic resonance images of the substantia nigra in Parkinson disease. Ann Neurol 71:267–277
Cosottini M, Frosini D, Pesaresi I et al (2014) MR Imaging of the Substantia Nigra at 7 T Enables Diagnosis of Parkinson Disease. Radiology 271:831–838
Duncan GW, Firbank MJ, O’Brien JT, Burn DJ (2013) Magnetic resonance imaging: a biomarker for cognitive impairment in Parkinson’s disease? Mov Disord 28:425–438
Nagano-Saito A, Washimi Y, Arahata Y et al (2005) Cerebral atrophy and its relation to cognitive impairment in Parkinson disease. Neurology 64:224–229
Beyer MK, Larsen JP, Aarsland D (2007) Gray matter atrophy in Parkinson disease with dementia and dementia with Lewy bodies. Neurology 69:747–754
Burton EJ, McKeith IG, Burn DJ et al (2004) Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain 127:791–800
Weintraub D, Dietz N, Duda JE et al (2012) Alzheimer’s disease pattern of brain atrophy predicts cognitive decline in Parkinson’s disease. Brain 135:170–180
Segura B, Baggio HC, Marti MJ et al (2014) Cortical thinning associated with mild cognitive impairment in Parkinson’s disease. Mov Disord 29:1495–1503
Melzer TR, Watts R, MacAskill MR et al (2012) Grey matter atrophy in cognitively impaired Parkinson’s disease. J Neurol Neurosurg Psychiatry 83:188–194
Scherfler C, Schocke MF, Seppi K et al (2006) Voxel-wise analysis of diffusion weighted imaging reveals disruption of the olfactory tract in Parkinson’s disease. Brain 129:538–542
Agosta F, Canu E, Stojkovic T et al (2013) The topography of brain damage at different stages of Parkinson’s disease. Hum Brain Mapp 34:2798–2807
Agosta F, Kostic VS, Davidovic K et al (2013) White matter abnormalities in Parkinson’s disease patients with glucocerebrosidase gene mutations. Mov Disord 28:772–778
Kwak Y, Peltier SJ, Bohnen NI et al (2012) L-DOPA changes spontaneous low-frequency BOLD signal oscillations in Parkinson’s disease: a resting state fMRI study. Front Syst Neurosci 6:52
Agosta F, Caso F, Stankovic I et al (2014) Cortico-striatal-thalamic network functional connectivity in hemiparkinsonism. Neurobiol Aging 35:2592–2602
Amboni M, Tessitore A, Esposito F et al (2015) Resting-state functional connectivity associated with mild cognitive impairment in Parkinson’s disease. J Neurol 262:425–434
Tessitore A, Esposito F, Vitale C et al (2012) Default-mode network connectivity in cognitively unimpaired patients with Parkinson disease. Neurology 79:2226–2232
O’Callaghan C, Hornberger M, Balsters JH et al (2016) Cerebellar atrophy in Parkinson’s disease and its implication for network connectivity. Brain 139:845–855
Baggio HC, Sala-Llonch R, Segura B et al (2014) Functional brain networks and cognitive deficits in Parkinson’s disease. Hum Brain Mapp 35:4620–4634
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
F Agosta serves on the editorial board of the Journal of Neurology and is Section Editor of NeuroImage: Clinical; and has received research supports from the Italian Ministry of Health, AriSLA—Fondazione Italiana di Ricerca per la Sclerosi Laterale Amiotrofica, and the European Research Council, and speaker honoraria from EXCEMED—Excellence in Medical Education. S. Galantucci reports no disclosures. M. Filippi is Editor-in-Chief of Journal of Neurology; serves on scientific advisory board for Teva Pharmaceutical Industries; has received compensation for consulting services and/or speaking activities from Bayer Schering Pharma, Biogen Idec, EXCEMED, Merck Serono, and Teva Pharmaceutical Industries; and receives research support from Bayer Schering Pharma, Biogen Idec, Merck Serono, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, Cure PSP, Alzheimer’s and Drug Discovery Foundation, and the Jacques and Gloria Gossweiler Foundation (Switzerland).
Rights and permissions
About this article
Cite this article
Agosta, F., Galantucci, S. & Filippi, M. Advanced magnetic resonance imaging of neurodegenerative diseases. Neurol Sci 38, 41–51 (2017). https://doi.org/10.1007/s10072-016-2764-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-016-2764-x