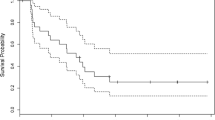
Abstract
Despite a confirmed survival benefit associated with adjuvant radio- and chemotherapy, the majority of patients with malignant glioma relapse after initial therapy. Recurrent malignant glioma treatment has not been standardised and usually the response rate to standard chemotherapy protocols for recurrent malignant glioma is less than 30%. The growing body of evidence demonstrating the clinical importance of O6-methylguanine methyltransferase (MGMT) has generated a considerable interest in the exploration of strategies to overcome MGMT-mediated resistance to alkylating agents; for example protracted administration of Temozolomide (TMZ) may result in more extensive and sustained depletion of MGMT; for this reason a variety of dosing schedules that increase the duration of exposure and the cumulative dose of TMZ are being investigated for the treatment of patient with recurrent malignant glioma after standard treatment. The most widely studied regimens in this setting include (1) 21 of 28-day schedule at a dose of 75–100 mg/m2/day; (2) 7 of 14-day schedule at a dose of 150 mg/m2/day, also referred to as the ‘‘one week on/one week off’’ schedule; (3) Continuous daily schedule at a dose of 50 mg/m2/day. An alternative dosing schedule of TMZ may be a reasonable option in patients having high-grade gliomas with recurrence after standard therapy.
Similar content being viewed by others
References
Stupp R, Mason WP, Van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Hegi ME, Diserens AC, Gorlia T et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003
Berrocal A, Perez Segura P, Gil M et al (2010) Extended-schedule dose-dense temozolomide in refractory gliomas. J Neurooncol 96:417–422
Franceschi E, Omuro AM, Lassman AB et al (2005) Salvage temozolomide for prior temozolomide responders. Cancer 104:2473–2476
Strick HM JH, Buhk JH, Wrede A et al (2008) Rechallenge with temozolomide with different scheduling is effective in recurrent malignant gliomas. Molecular Medicine Reports 1:863–867
Perry JR, Bélanger K, Mason WP et al (2010) Phase II trial of continuous dose-intense temozolomide in recurrent malignant glioma: RESCUE study. J Clin Oncol 28(12):2051–2057
Wick A, Pascher C, Wick W et al (2009) Rechallenge with temozolomide in patients with recurrent gliomas. J Neurol 256:734–741
Tosoni A, Cavallo G, Ermani et al (2006) Is protracted low-dose temozolomide feasible in glioma patients? Neurology 66:427–429
Neyns B, Chaskis C, Joosens E et al (2008) A multicenter cohort study of dose dense temozolomide (21 of 28 days) for the treatment of recurrent anaplastic astrocytoma or oligoastrocytoma. Cancer Invest 26:269–277
Conflict of interest
The authors declare that there is no actual or potential conflict of interest in relation to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gaviani, P., Silvani, A., Lamperti, E. et al. Rechallenge with temozolomide in recurrent glioma. Neurol Sci 32 (Suppl 2), 247–249 (2011). https://doi.org/10.1007/s10072-011-0798-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-011-0798-7