Abstract
Apes, human’s closest living relatives, are renowned for their intentional and highly flexible use of gestural communication. In stark contrast, evidence for flexible and intentional gestural communication in monkeys is scarce. Here, we investigated the intentionality and flexibility of spontaneous gesture use in red-capped mangabeys (Cercocebus torquatus). We applied established methods used in ape gesture research to analyse whether the body acts produced by a total of 17 individuals living in three different groups in captivity qualified as intentionally produced gesture instances. Results showed that signallers showed all hallmarks of intentionality during the production of 20 out of a total of 21 different types of body acts. These were only produced in the presence of other individuals, and the monkeys showed audience checking, sensitivity to the attentional states of recipients, adjustment of signal modality, and response waiting relative to their production. Moreover, in case of communication failure, the monkeys showed goal persistence, and regarding the production contexts they showed some signs of means–ends dissociation. Therefore, these monkeys are capable of flexible and intentional gestural communication and use this to communicate with conspecifics. Our results corroborate recent findings showing that intentional gestural communication was already present in the monkey lineage of catarrhine primates. We discuss our results in light of the comparative approach towards human language evolution and highlight our finding that these monkeys also showed flexible and intentional use of four ‘free’ manual gesture types.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Human language is a unique communication system in the animal kingdom, which crucially depends on a complex interplay of several sensory–motor, cognitive and computational capacities (Hauser et al. 2002). Trying to unravel its evolutionary roots has been a primary interest of researchers studying non-human animal communication and human language evolution in past decades (e.g. Hewes 1973; Snowdon et al. 1982; Seyfarth 2005; Arbib et al. 2008; Tomasello 2010; Petkov and Jarvis 2012; Lemasson et al. 2013; Fitch 2017). In this context, past comparative research revealed that precursors or pre-adaptations for several cognitive abilities needed for language can be found in non-human animal communication systems (Hockett 1959; Fitch 2010; Hauser et al. 2002). One of these is the ability to communicate intentionally, which requires from individuals involved in the communicative act a certain degree of intentionality. In cognitive and philosophical sciences, intentionality has been described as the capacity of mental states (e.g. knowing, believing and desiring) to be ‘about’ objects, properties, or situations (including other mental states), or to ‘refer’ to them (reviewed in Searle 1983; Dennett 1983). Although originally a purely philosophical concept considered only applicable to mental phenomena, it has subsequently been applied to explain the intentionality of behavioural acts, such as communicative behaviour in humans (Grice 1957) and animals (Premack and Woodruff 1978; Dennett 1983).
In particular, Dennett’s (1983) framework for considering the degree of intentionality underlying animal behaviour has become the topic of reheated interest and debates in recent comparative communication studies (see, e.g. Scott-Philips 2015; Moore 2016; Fischer and Price 2016; Townsend et al. 2017; Graham et al. 2020; Ben Mocha and Burkart 2021). According to this framework, different levels of intentionality may underlie animal communicative behaviour, ranging from communication with ‘zero-order intentionality’ at one extreme, to overtly intentional or ‘ostensive’ communication at the other. In communication with zero-order intentionality, any form of information transfer between a signaller and a recipient occurs as a result of the signaller’s and recipient’s automated responses to environmental cues. For this, the individuals neither need to have a mental state of their own, nor to recognise the consequences of their behaviour on the behaviour or mental state of others, i.e. no sophisticated mental processes are required. In stark contrast, for ostensive communication complex mental processes are required, with both signaller and recipient recognising each other’s mental states and behaving accordingly (Grice 1957; Dennett 1983; Sperber and Wilson 1995; Scott-Philips 2015). This latter type has traditionally been regarded as ‘true communication’ and is common in human language (Grice 1957).
The aforementioned scientific debate in comparative communication studies revolves around questions related to the attribution of mental states to animals, i.e. whether any non-human animal has mental states at all, whether they can know them, share them, or manipulate them, whether and how it is possible to measure them, and whether animals would actually need (mutual) mind-reading for successful communicative exchanges (Scott-Philips 2015; Fischer and Price 2016; Moore 2016; Fitch 2016; Townsend et al. 2017; Tomasello and Call 2019; Leavens et al. 2019). Yet, at the same time, non-human animals are increasingly deemed capable of more than mere automated communicative behaviour, with in particular apes having been widely acknowledged to use a form of communication that lies somewhere in between zero-order intentional and ostensive communication, i.e. ‘first-order intentional communication’ (Dennett 1983; Call and Tomasello 2007). This is voluntary (rather than automated) and socially directed (rather than indiscriminately broadcast) communicative behaviour that is produced in a goal-directed way, i.e. with the explicit goal of changing a recipient’s behaviour in a particular way (Dennett 1983; Tomasello 2010; Townsend et al. 2017; Byrne et al. 2017). It is the voluntary and socially directed production that defines this behaviour as deliberately communicative, and that discriminates it from mere informative behaviour that may convey messages without being voluntarily emitted by the signaller to anyone in particular. The goal-directed production, in addition, may reveal something more about the signaller’s goals and intentions underlying its behaviour and its ability to recognise the effects of its own behaviour on the behaviour of other individuals. Following Dennett (1983), these combined capacities could be interpreted as the signaller having a mental state of its own, making it a ‘first-order intentional system’ that is communicating with first-order intentionality.
As mentioned, however, in most animal communication studies it has been common to remain more conservative in interpreting animal communicative behaviour in terms of underlying mental states. Instead, an animal’s deliberate and goal-directed production of communicative acts is often interpreted in leaner ways, e.g. in terms of their apparent ultimate function in relation to the behavioural context in which they occur, or in terms of the ‘apparently satisfactory outcomes’ (ASO’s) of the signalling behaviour that can be determined from the behavioural interplay between signallers and their recipients (Genty et al. 2009; Cartmill and Byrne 2010; Hobaiter and Byrne 2014; 2017; Scott-Phillips 2015; Byrne et al. 2017; Molesti et al. 2020). The latter is considered to be a purely behavioural proxy for the signaller’s ‘intended meaning’ (Genty et al. 2009; Cartmill and Byrne 2010; Hobaiter and Byrne 2014, 2017). Hence, if anything were to be determined at all about potential mental states underlying the animals’ communicative behaviour from such studies, it can at most be concluded that the signaller behaves as if communicating with a particular goal in mind (Scott-Phillips 2015; Fischer and Price 2016; Hobaiter and Byrne 2017; Leavens et al. 2019).
Similarly, the methodology used to distinguish between communication with first-order intentionality and mere automated forms of communication has also been designed to largely avoid the question of mental state attribution, and is based on pure behavioural proxies that, across scientific disciplines, have been agreed to be indicative of a signaller’s communicative and goal-directed intentions during social interactions. These behavioural proxies (‘markers’) were originally defined in child development studies investigating the intentionality of non-verbal communication in pre-linguistic children (Bates et al. 1975) and were later adapted to be used in primate research (Tomasello et al. 1994, 1997; Pika et al. 2003; Leavens et al. 2004, 2005; Call and Tomasello 2007; Townsend et al. 2017). Following these definitions, to determine whether or not a communicative event between a signaller and recipient is the product of an intention on behalf of the signaller, it first of all needs to be assessed if a signaller’s behaviour is produced in a voluntary and socially directed way that is conducive to successful communication. This is the case if the behaviour is produced in the presence rather than absence of an audience, is preceded by audience checking (signaller monitors its audience before performing its behaviour and orients to a recipient), and is associated with a signaller’s sensitivity to the attentional state of its recipient (signaller takes into account whether the recipient can perceive its behaviour and adjusts its behaviour if it cannot). Evidence that the behaviour is produced in a goal-directed manner is provided when the signaller shows signs of response waiting after producing it (signaller pauses its behaviour for a given period of time, during which he maintains visual contact, apparently monitoring the recipient’s behaviour), and signs of goal persistence, a flexible behavioural strategy that is contingent on the recipient’s response. Hence, in cases where a signaller’s apparent goal, i.e. a particular behaviour change from the recipient, has been reached it will stop further signalling behaviour, whereas in cases where this goal has not yet been reached, the signaller will perform further signalling behaviour. Furthermore, the use of ‘means–ends dissociation’ (Bruner 1981), in which one signal type is used flexibly in several behavioural contexts and/or several signal types are used in the same context interchangeably towards the same end, is often taken as complementary evidence that the communication is goal directed and intentional (Plooi 1978, 1984; Tomasello et al. 1985, 1997; Liebal et al. 2004; Pika et al. 2003, 2005a; Cartmill and Maestripieri 2012; Fischer and Price 2016; Molesti et al. 2020). And, finally, the use of gaze alternation (signaller alternates its gaze between the recipient and an external object or event of interest) during signal production is considered evidence that the signaller is communicating ‘about’ something to its recipient (Bard 1992; Leavens and Hopkins 1998, Leavens et al. 2004; 2005; Bard et al. 2014; but see Tomasello and Call 2019 for a critical account about this). Together, these ‘markers of intentionality’, thus, specifically focus on assessing both the communicative and goal-directed aspects of an animal’s behavioural acts, with a particular focus on the signallers’ flexibility in obtaining their communicative goals.
Systematic assessment of animals’ use of these markers of intentionality underlying their communicative behaviour has, so far, revealed that the gesture production of all ape species can be qualified as first-order intentional communication (reviewed, e.g. in Byrne et al. 2017; Tomasello and Call 2019; see Schel et al. 2013 for the first evidence of intentional use of vocal communication in chimpanzees). Gestures are a subset of communicative signals that have been defined as recipient-directed movements of the hands, feet, limbs, head, or body postures, which are mechanically ineffective (i.e. they are not designed to act as direct physical agents) and result in a voluntary response from the recipient (e.g. Plooij 1978; Pika 2008; Call and Tomasello 2007; Pika and Liebal 2012; Liebal and Call 2012). The a priori element of recipient directedness defining a gesture already distinguishes gestures from merely automated and indiscriminately broadcast body acts and classifies them per definition as deliberately communicative behaviour. Hence, ape gestures are produced in the presence of an audience (Call and Tomasello 1994; Hostetter et al. 2001; Leavens et al. 2004), with their production generally being preceded by audience checking (Genty et al. 2009; Graham et al. 2017; Hobaiter and Byrne 2011), and signallers showing a sensitivity to the attentional state of their recipient when producing their gestures (Call and Tomasello 1994; Hostetter et al. 2001; Leavens et al. 2004; Pika et al. 2005b; Liebal et al. 2006; Genty et al. 2009). Furthermore, ape gesture production is goal directed, with signallers consistently showing response waiting after producing their gestures (Genty et al. 2009; Hobaiter and Byrne 2011) and clear signs of goal persistence in cases where the presumed communicative goal was not met (Cartmill and Byrne 2007; Leavens et al. 2005; Roberts et al. 2014). Moreover, apes have been found to show flexibility in their gesture production in terms of ‘means–ends dissociation’ (Tomasello et al. 1994; Pika et al. 2005b; Liebal et al. 2006; Genty et al. 2009; Graham et al. 2017), as well as gaze alternation in combination with gesture use in cases where a third entity was involved in the communicative act (Bard 1992; Leavens and Hopkins 1998). These findings have made apes’ gestural communication excellent examples of first-order intentional communication in the animal kingdom, which, subsequently, have been exerting a strong and continued influence on theories on human language evolution (e.g. Arbib et al. 2008; Tomasello and Call 2019; Ben Mocha and Burkart 2021).
In fact, the continued focus on, and fine-tuning of, ape gesture studies has led to some increasingly specific ideas concerning their merit for theories on human language origins (see Cartmill and Hobaiter 2019; Leavens et al. 2019 for discussions about this). An example comes from ape studies focussing specifically on ‘free’ brachio-manual gestures, i.e. gestures only produced with the hands, feet or limbs, without making contact with a substrate or partner (Pollick and de Waal 2007; de Waal and Pollick 2011; Roberts et al. 2012, 2014, 2019). Because of the myriad of contexts the hands are generally used in, free manual gesture production has been considered to be even more flexible than the production of other gesture types (Call and Tomasello 2007). This flexible use of free manual gestures in the apes, in combination with a reported ‘virtual absence’ of such gesture production in monkey species (de Waal 2003; Pollick and de Waal 2007; de Waal and Pollick 2011; Cartmill and Maestripieri 2012; Roberts et al. 2014), has been taken to suggest that the ‘shift toward a more flexible and intentional communicative strategy’ in our ancestors occurred relatively recent, i.e. in the hominoid lineage (sensu Pollick and de Waal 2007; de Waal 2003). However, the necessary comparative work on monkeys’ intentional and manual gesture use to support this idea is currently still largely lacking. To gain a full understanding of both the occurrence and form of intentional gestural communication in the primate lineage and its potential implications for theories on language origins, an equally fine-grained and systematic investigation of monkeys’ gestural communication is needed. The main objective of our study was, therefore, to investigate if monkeys make use of similar forms of intentional gestural communication as found in the apes.
In this respect, several previous studies have already focused on monkeys’ gesture production in experimental setups. These studies showed that some monkeys, like apes, adjust their trained manual gesture production to the presence (Cercocebus torquatus: Aychet et al. 2020) and attentional states of human experimenters (Cebus apella: Hattori et al. 2007, 2010; Defolie et al. 2015; Saimiri sciureus: Anderson et al. 2010; Cercocebus torquatus: Maille et al. 2012; Aychet et al. 2020; Papio anubis: Meunier et al. 2013; Bourjade et al. 2014; Molesti et al. 2020; Macaca tonkeana: Canteloup et al. 2015a; Macaca mulatta: Canteloup et al. 2015b), indicating the signallers’ communicative intent. Some of these studies also provided evidence for the use of gaze alternation during gesture production (Anderson et al. 2010; Meunier et al. 2013; Bourjade et al. 2014; Canteloup et al. 2015b; Aychet et al. 2020), suggesting that signallers were communicating ‘about’ something to their recipients. Yet, only few studies have focussed on the flexibility of gesture use in terms of goal persistence (Macaca radiata: Gupta and Sinha 2019; Cercocebus torquatus: Aychet et al. 2020) and means–ends dissociation (Macaca nemenstrina: Maestripieri 1996; Macaca sylvanus: Hesler and Fischer 2007; Papio anubis: Molesti et al. 2020), despite these being considered strong complementary markers of intentional production (Bates et al. 1975; Bruner 1981; Leavens et al. 2005) that may indicate a signaller is producing its communicative behaviour with a particular goal ‘in mind’ (Dennett 1983; Hobaiter and Byrne 2017; Byrne et al. 2017). This lack of data likely relates to the fact that most of the aforementioned studies have predominantly focused on monkey gesture use in experimental setups, in which the subjects were exclusively trained to produce one particular gesture type in one particular context to human experimenters. To date, only few monkey studies have focused on spontaneous and intraspecific intentional gesture production in non- experimental settings that may leave more room for observing flexibility in terms of goal persistence and means–ends dissociation (Maestripieri 1996; Hesler and Fischer 2007; Gupta and Sinha 2019; Molesti et al. 2020).
In this study, therefore, we particularly focussed on determining whether or not captive red-capped mangabeys (Cercocebus torquatus), a monkey species of the cercopithecoid superfamily of catarrhine primates that has been used as a model species in both vocal and gestural communication studies before (Maille et al. 2012; Bouchet et al. 2013; Aychet et al. 2020), made intentional and flexible use of gestures during their communicative interactions with conspecifics. We did this rigorously and from scratch, by assessing whether they showed all markers of intentionality during the spontaneous production of any of their potentially communicative body acts. A second aim was to find out whether the monkeys, if found to use intentionally produced gestures during their intraspecific interactions, also made use of free manual gesture types, i.e. gestures only produced with the hands, feet or limbs, without making contact with a substrate or partner. Given the fact that in previous studies red-capped mangabeys have been found to use trained manual gesture types in intentional ways towards human experimenters (Maille et al. 2012; Aychet et al. 2020), we expected the monkeys to be capable of intentional and manual gestural communication amongst conspecifics. The results of this study will contribute to a growing body of research towards the intentionality of monkey gestural communication that is needed for a more complete understanding of the spread of this capacity throughout the primate lineage and its potential implications for theories on human language origins.
Methods
Study site and subjects
We collected focal video footage from a total of 17 adult and subadult captive red-capped mangabeys living in three social groups at the Station Biologique de Paimpont, France (Table 1). Red-capped mangabeys are medium sized (7.5–10 kg) diurnal and predominantly terrestrial primates that naturally live in multimale-multifemale groups (Gautier-Hion et al. 1999; Jones and Sabater-Pi 1968; Cooke 2012; Orimaye 2017). Individual females and males are classified as adults when they are > 4 and 7 years of age, respectively, and as subadults when they are between 3 and 4 (females) or 7 (males) years of age, based on demographic data on a closely related species, i.e. grey-cheeked mangabeys (Lophocebus albigena; Chalmers 1968; Gautier-Hion and Gautier 1976; Deputte 1992).
Each individual had access to one outdoor enclosure and one indoor enclosure with different sizes (ranging from 15.3 to 29 m2), which were connected by tunnels. The different enclosures for the groups were subdivided into several sub-enclosures separated by gratings. The monkeys could move freely between the inside and outside enclosures, except during keepers’ cleaning activities. The floor of the indoor enclosures was covered by straw and sawdust, whereas the floor of the outdoor enclosures was covered by bark or cement. Enrichment consisted of beams, ropes, chains, and tires. In the indoor enclosures, the temperature was stabilised at 22 ± 2 °C. The monkeys were fed twice daily with fresh vegetables and fruits in the morning and monkey chow in the afternoon. Fresh water was available ad libitum.
Data collection
In February 2015, daily observations took place between 9 a.m. and 5 p.m. on a total of 13 observation days, with the monkeys being observed for a total of 4–6 h per day. Independent 15 min. continuous focal-animal sampling (Altmann 1974) was used to video record the behaviour of the 17 adult and subadult focal individuals for a total duration of 120 min per individual, using a Panasonic HC-X920 digital video camera. Focal animals were selected following a preset randomised order. Usually, the focus was on individuals from one group in the morning (between 9h:00 and 12h:00) and another in the afternoon (between 13h:00 and 17h:00), and the next day this order was alternated. If a focal subject moved outside the range of vision, the recording was stopped, but the recorded focal time was added to the total sampling time for that individual if it had lasted at least one minute. If a focal subject did not return within one minute after moving out of sight, the next session with a new focal animal was started. Comments were given on the general behaviour of both the focal individual and their potential recipient, their identity and sex, identities of other animals present within the same area of the enclosure, and whether or not an external entity appeared to be involved in the communicatory act. The video recordings were transferred to a PC and then loaded into Solomon Coder 15.1.13.0 for further video analyses.
Identifying intentional gestural communication
In this study, the body acts that were further analysed for their potential to be defined as intentionally produced gestures comprised all instances of brachio-manual movements, leg movements, whole body movements, and body postures other than those performed during general locomotion, feeding, exploration, foraging and self-directed activities, and included those where non-mechanically effective physical contact with any other individual or a substrate was made.
To determine from the video recordings whether or not these body acts qualified as intentionally produced gestures, we first of all assessed whether they were ‘potentially communicative’. For this, we checked whether the monkeys were more likely to produce them in the presence rather than absence of other individuals (see Table 2 for operational criteria). For each single body act instance produced in the presence of other individuals, we subsequently determined whether the monkeys showed signs of audience checking and sensitivity to the attentional state of the recipient during their production (see Table 2 for operational criteria). To determine whether the production of these body acts was also associated with signs of goal-directedness, we additionally recorded for each body act instance whether or not the signallers showed signs of response waiting after producing it, which type of voluntary response was shown by the recipient, and whether or not the signaller showed any particular signs of goal persistence in case of an (apparently) unsatisfactory response from their recipient (see Table 2 for operational criteria). Recipients’ voluntary responses were scored as 1. ‘response’, i.e. a behaviour change that could occur in two forms: (a) recipient stops its current activity within a particular behavioural context and changes it to one within a different behavioural context, and (b) within one behavioural context the recipient changes its focus from self-directed behaviour or interacting with another individual than the signaller to interacting with the signaller, or 2. ‘no response’, i.e. the recipient does not show a behaviour change. Behavioural contexts identified were: grooming (including e.g. start/stop grooming, change of grooming position), agonistic (including aggression and submission), affiliative (social positive behaviours other than grooming), sexual (e.g. copulation, inspect or touch genitals), social play, and other (any behavioural context other than the aforementioned contexts, e.g. responding to keeper’s presence). Because a preliminary inspection of the monkeys’ interactions revealed that none of the signallers’ body acts concerned an external object or third party, the marker ‘gaze alternation’ was omitted from further analyses.
Identifying gesture types
Only those body act instances associated with all markers of intentionality (apart from gaze alternation) were considered intentionally produced gesture instances (see, e.g. Townsend et al. 2017; Ben Mocha and Burkart 2021). These included the instances where signallers stopped producing further body acts after receiving an apparently satisfying response from their recipient (i.e. an ‘intended’ behaviour change), as well as the instances where they continued producing body acts after receiving an apparently unsatisfactory response from their recipient (i.e. either no behaviour change at all or an ‘unintended’ behaviour change). Instances where the signallers did not produce any further body acts after receiving no behaviour change from their recipient were not included, as in this latter case it could not be assessed whether the signaller appeared to have had an initial goal or not (see, e.g. Townsend et al. 2017). All intentionally produced gesture instances were subsequently described in behavioural terms and classified as instances of a particular gesture type. These gesture types were then further categorised within one of the following gesture categories (Pika et al. 2003): (i) visual only gestures, i.e. distant signals representing movements of hands, body parts, or body postures, (ii) tactile gestures, i.e. visual signals that involve physical contact between the interacting animals (e.g. embrace), and (iii) audible gestures, i.e. visual signals that produce a sound during their production (e.g. a ground slap). Together, these gesture types represented the preliminary gestural repertoire of the monkeys in this study group. Using this preliminary gestural repertoire, we finally determined whether the mangabeys made use of ‘free’ manual gesture types as well, by checking which gesture types were produced by making movements of the arms and/or hands only, without making physical contact with a substrate or partner (de Waal 2003; Roberts et al. 2012, 2014). Note here that we decided to include all intentionally produced gesture types in the description of the monkeys’ preliminary gestural repertoire, also if they did not fulfil the often used requirement of having to have been used for an overall minimum of two times by at least two different individuals, or at least five times by one individual, to be included in the repertoire (e.g. Pika et al. 2003; Tomasello et al. 1985; Liebal et al. 2004; Hobaiter and Byrne 2011; Graham et al. 2016). This decision was made following a preliminary inspection of the gesture instances produced by non-focal individuals that were also caught on camera during focal video recordings, which revealed that the majority (85%) of gesture types produced by the focal individuals and reported in the preliminary gestural repertoire were also produced by other, non-focal, individuals in intentional ways (see Table 4 for details).
Identifying flexibility of use
To assess the monkeys’ flexibility of gesture use in terms of means–ends dissociation, we determined the variety of behavioural contexts in which each particular gesture type was used. For this, the pre- and post-contextual information accompanying the signaller’s gesture production was analysed, with a focus on the ensuing behavioural change in the recipient or the immediately ensuing behavioural interaction between signaller and recipient following gesture production (Tomasello et al. 1997; Liebal et al. 2004). Depending on the nature of this assessment, we defined five contexts in which gestures were used: affiliative, agonistic, grooming, play, sexual. Instances of which the context remained ambiguous from the videos were coded as ‘unclear’.
Inter-observer reliability
Inter-observer reliability coding of the occurrence of all intentionality criteria, as well as the classification of gesture types and context of use, was performed by a second observer for 25% of the data. For the intentionality criteria we used Cohen’s kappa (κ) to measure the degree of concordance between the two observers, and for gesture type and context we simply calculated the percentage of agreement. ‘Substantial’ to ‘excellent’ levels (McHugh 2012) of inter-observer agreement were found for all measured criteria (κ gesture category = 0.75, 89% agree; κ attentional state recipient = 0.74, 97% agree; κ audience checking = 1.0, 100% agree; κ response waiting = 0.90, 96% agree; κ change of recipient behaviour = 0.85, 95% agree; κ goal persistence = 0.72, 90% agree), as well as for gesture type (92% agree) and context of use (83% agree). The instances for which inconsistencies existed between observers were double checked and discussed between observers, after which it was decided to keep the original coder’s coding for all further instances, as these turned out to be scored most accurately.
Statistics
We used R. 3.5.0 software (R Core Team 2018) to conduct statistical tests. Because of our relatively small sample size (N = 17 individuals), we mainly used descriptive statistics and non-parametric statistical tests. All tests were two tailed, with the alpha-level set at 0.05. The presence of each intentionality marker associated with the production of body acts was studied first at the level of body act occurrences (lumping all instances together), using tests for proportion comparisons (binomial and Chi Square tests), except for the first marker (presence vs absence audience), for which a ratio test was used. Then we also tested this at the individual level whenever possible, using Wilcoxon signed ranks tests, to account for potential inter-individual differences in signalling.
Regarding the marker ‘presence/absence audience’, we compared the monkeys’ rates of body act production between social vs alone conditions using a ratio test (Sahai and Kursid 1996; Martin and Austin 1996). Because of the small number of potentially communicative body acts produced in the alone condition, this was only possible on the group level. Regarding the marker ‘audience checking’, we assessed the likelihood of signallers’ body acts being associated with a signaller looking at the recipient prior to producing it. For the marker ‘sensitivity to the attentional state of the recipient’, we first tested whether or not body acts were preferentially produced in the presence of a visually attending or non-attending recipient. Moreover, we compared the proportion of each signal category (visual only or non-visual only, i.e. audible or tactile) depending on recipient’s attentional state. To run this analysis also at the individual level, we only kept the data of individuals that produced body acts both in the presence of attending and non-attending recipients (N = 7). Then, we tested the likelihood of body acts being associated with the marker ‘response waiting’. Regarding the marker ‘goal persistence’, we determined whether the form of goal persistence (stop signalling/repeat same signal/produce new signal) depended on the type of response by the recipient (response or no response). To run this test at the individual level as well, we only kept data of individuals that received both ‘responses’ and ‘no responses’ from their recipients (N = 13).
Finally, with regards to measuring means–ends dissociation, a preliminary inspection of our data revealed that the two hours of observation per individual provided insufficient data to address this question statistically. Therefore, we simply counted the number of different gesture types used within the same broad behavioural context, as well as the number of different contexts in which one particular gesture type was used (Pika et al. 2005b; Liebal et al. 2006; Call and Tomasello 2007; Genty et al. 2009). These were reported descriptively to get a first impression of the variety of contexts in which the particular gesture types were used.
Results
Intentionality of use
Presence/absence audience
From the 2040 min of focal observation (N = 17 individuals, 120 min per individual), the monkeys were observed to be ‘alone’ for a total of 132 min and ‘social’ for a total of 1908 min. In the social condition, the monkeys produced a total of 297 body acts not usually performed during general locomotion, feeding, foraging, exploration and self-directed activities (range = 1–40 per individual; median = 16), whereas in the alone condition they produced a total of five instances of such body acts. The monkeys were, thus, four times more likely to produce these body acts in the social condition compared to the alone condition (rate in social condition = 15.57; rate in alone condition = 3.788; RateRatio = 4.109; 95% CI = 1.70–9.90; χ2 = 11.7; exact mid-p = 0.00009).
Because the five instances of non-socially produced body acts were not potentially communicative to begin with, we only used the socially produced body acts (N = 297) to further assess whether their production was associated with each of the additional markers of intentional communication.
Audience checking
From the total of 297 instances of socially produced body acts, the majority (293; 99%) were preceded by audience checking (Binomial test: N = 297, Pexp = 0.50, Pobs = 0.99, p < 0.001). This effect was also found at the individual level. All 17 individuals showed audience checking, with the mangabeys visually orienting to their recipient before producing their behaviour in the majority of cases (Wilcoxon signed rank test: N = 17, V = 153, p < 0.001).
Sensitivity to the attentional state of the recipient
For two out of the 297 socially produced body act instances, the attentional state of the recipients could not be determined due to insufficient recording conditions. From the remaining 295 instances, the majority (280; 95%) were produced by the signallers when recipients were visually attending (Binomial test: N = 295, Pexp = 0.50, Pobs = 0.95, p < 0.001). This effect was also found at the individual level. All 17 individuals showed a sensitivity to the attentional state of their recipient, with the mangabeys generally producing body acts more often in front of visually attending compared to non-visually attending recipients (Wilcoxon signed rank test: N = 17, V = 136, p < 0.001).
From the 280 instances produced to visually attending recipients, 234 (84%) were visual only, 42 (15%) were tactile and four (1%) were audible. From the 15 body act instances that were produced when recipients were not visually attending, six (40%) were visual only, eight (53%) were tactile and one (7%) was audible. Further analysis of these results showed that there was a significant association between the attentional state of the recipient and the signal category used by the signallers. Signallers used visual signals less often and non-visual signals more often than expected by chance when recipients were not visually attending (χ2 (1, N = 295) = 17.82; p < 0.001), despite some variation in the use of gesture categories to non-visually attending recipients at both the group level (Binomial test: N = 15, Pexp = 0.50, Pobs = 0.40, p = 0.607) and the individual level (Fig. 1, Wilcoxon signed rank test: N = 7, V = 12, p = 0.829).
Response waiting
From the total of 297 socially produced body act instances, the majority (285; 96%) were followed by response waiting (Binomial test: N = 297, Pexp = 0.50, Pobs = 0.96, p < 0.001). This effect was also found at the individual level. All 17 individuals showed response waiting after gesture production, with the mangabeys monitoring a recipient’s behaviour after producing body signals in the majority of cases (Wilcoxon signed rank test: N = 17, V = 153, p < 0.001).
Goal persistence
A total of 104 (35%) out of all 297 socially produced body act instances resulted in ‘no response’ from the recipients (i.e. no behaviour change at all) and 193 (65%) out of the 297 instances led to a response (i.e. a behaviour change). From the 104 instances leading to no response, signallers stopped their body act production in 50 (48%) cases and continued producing further body acts in 54 (52%) cases. From the 193 instances leading to a response, the signallers stopped further body act production in 127 cases (66%) and continued with further body act production in 66 cases (34%).
In case of an apparently unsatisfactory response from the recipient (i.e. either a non-intended behaviour change or no behaviour change at all), 13 of the 17 individual signallers showed further body act production in the form of persistence and eight of the 17 individual signallers showed further body act production in the form of elaboration. The form of this further signalling behaviour was contingent on the type of unsatisfactory response they had received from their recipients. When the monkeys continued further body act production after receiving no behaviour change at all (N = 54), they persisted with the same type of body act behaviour in 39 cases (72%) and elaborated their initial body act behaviour by using a different type in 15 cases (28%). If they continued further body act production after receiving an apparently non-intended behaviour change (N = 66), they elaborated their body act behaviour in 38 cases (58%) and persisted with the same type in 28 cases (42%). Further analysis of these results showed that there was a significant association between the type of recipient response and the signaller’s production of further body acts. The monkeys were more likely than expected by chance to stop producing further body acts after having received a response from their recipient compared to having received no response (N = 297, χ2 = 8.8191, df = 1, p value = 0.003), and to repeat the same body act behaviour (persist) as further signalling behaviour in cases where they received no response from their recipient compared to having received an apparently wrong response; in the latter case they also often decided to elaborate their communicative behaviour by using a different body act (N = 120, χ2 = 10.694, df = 1, p value = 0.001). The same effect was also found at the individual level (Fig. 2A and B, Wilcoxon signed rank test: persistence after response: N = 13, V = 24, p = 0.438; persistence after no response: N = 13, V = 10, p = 0.041).
A Proportions of further body act production (‘further signalling’) or no further body act production (‘no further signalling’) by signallers in relation to receiving either a response or no response from their recipient (N = 15 individuals). B Proportions of elaboration or persistence as further signalling behaviour by signallers in relation to receiving a response or no response from their recipient (N = 13 individuals). Wilcoxon signed rank tests: *p ≤ 0.050; ns: p > 0.050
In total, 243 out of the 297 socially produced body act instances (82%) were each associated with all the aforementioned markers of intentionality (see Table 4) and classified as intentionally produced gesture instances. These were further used to describe the different gesture types produced by these monkeys and, by extension, the preliminary gestural repertoire of this study population.
Description of gesture types
From the total of 243 intentionally produced gesture instances, we identified 20 different gesture types (Table 3). These were all produced at least once by at least one individual in concordance with all markers of intentionality (Table 4). Moreover, for all gesture types except ‘roll on ground’, ‘salto’, and ‘swing body’ there were observed cases of at least one additional non-focal individual producing this gesture type intentionally as well (Table 4). One other observed body act, ‘shake object’, was not accompanied by all markers of intentionality and was also not produced by any other non-focal individual. This body act was, therefore, not included as a gesture type in the preliminary gestural repertoire (Table 4).
Four of the 20 gesture types were categorised as ‘tactile’ gestures, two were categorised as ‘audible’ gestures, and 14 were categorised as ‘visual only’ gestures. Of the 14 visual only gesture types, four were ‘free’ manual gesture types (gesture types 6, 7, 12 and 14 in Table 3), i.e. produced by making movements of the arms and/or hands only, without making physical contact with a substrate or partner (sensu de Waal 2003; Roberts et al. 2012, 2014).
Flexibility of use
With regards to flexibility of gesture use in terms of ‘means–ends dissociation’, we described the different contexts in which the different gesture types were used across all individuals (Table 5). Two manual gesture types (‘throw arm towards’ and ‘throw hand towards’) and three non-manual gesture types (‘hit body part’, ‘put body part on other’ and ‘present body part’) were used flexibly, i.e. in both the social play context and at least one additional behavioural context (i.e. agonism or grooming, with ‘present body part’ being produced in three contexts additional to play, i.e. groom, affiliative and sexual). In addition, the monkeys made use of several different gesture types within the same functional context for both the play context (13 different gesture types used) and agonistic context (nine different gesture types used).
Discussion
Intentionality of gesture use
In this study, we investigated whether the intraspecific production of potentially communicative body acts of a group of captive red-capped mangabeys qualified as flexible and intentional gestural communication, and whether individuals used ‘free’ manual gestures during their communicative exchanges as well. The mangabeys produced a total of 21 types of body acts that were not usually produced as more general locomotion, foraging, exploration and self-directed activities. These were all mechanically ineffective and led to a voluntary response by recipients. Of these, 20 were classified as intentionally produced gesture types, as their production fulfilled specific markers characterising intentional communication. These gesture types were generally (i) produced in the presence of recipients, (ii) preceded by audience checking, (iii) adjusted to the attentional state of the recipient, (iv) followed by response waiting, and (v) accompanied by signs of goal persistence. More specifically, in terms of attentional state adjustment, the mangabeys used visual only gesture types more frequently in situations with visually attentive recipients, and tactile and audible gesture types in situations with non-visually attentive recipients. Concerning goal persistence, they often continued producing further body acts if their initial behaviour was followed by an apparently unsatisfactory response from the recipient. Red-capped mangabeys, thus, appear able to communicate in intentionally communicative and goal-directed ways with their conspecifics.
These results corroborate findings of two recent studies providing evidence for intraspecific intentional gesture production in two other catarrhine monkey species, wild bonnet macaques (Gupta and Sinha 2019) and captive olive baboons (Molesti et al. 2020). The study on the bonnet macaques paid special attention to the markers response waiting and goal persistence during gestural interactions between already attentive communicative partners, while the study on the olive baboons examined the markers audience checking, attentional state adjustment, and response waiting. Our study adds to the growing body of evidence that first-order intentional communication may be a more universal pattern in primate communication, by providing an assessment of the gestural communication of a single group of monkeys with regards to all markers of intentionality. Due to this choice of markers, it conquers recent debates concerning the validity of using only one or a restricted set of markers when assessing animals’ intentional communication skills (see, e.g. Liebal et al. 2013; Townsend et al. 2017; Graham et al. 2020; Ben Mocha and Burkart 2021; Rodrigues et al. 2021). The different markers of intentionality jointly assess several aspects of both the communicative and goal-directed properties of socially produced behaviour, with each marker contributing its own added value to this assessment (Ben Mocha and Burkart 2021). Ideally, therefore, any study claiming that an animal’s apparently communicative behaviour is ‘intentional’ should clearly account for both the communicative and goal-directed aspects of this behaviour, by providing converging evidence for the use of as many markers of intentionality as possible (Liebal et al. 2013; Townsend et al. 2017; Graham et al. 2020; Ben Mocha and Burkhart 2021) and providing as much detail as possible on their frequency and distribution of use (Rodrigues et al. 2021; Ben Mocha and Burkart 2021).
According to several scholars (e.g. Bard 1992; Leavens et al. 1996; Bard et al. 2014), this includes assessing the use of gaze alternation accompanying signal production that is directed towards an external entity, as this could provide evidence that an animal is capable of communicating ‘about’ something to its recipient. Yet, such triadic communicative interactions are rare to observe in natural settings, as the majority of communicative interactions across adult primates occur as dyadic events, in which signallers merely aim to direct the behaviour of their recipients relative to their own social motivations and goals (Hurford 2007; Liebal et al. 2013; Tomasello and Call. 2019). Consequently, this is the one marker that is most often missing or omitted in studies towards intentional communication amongst conspecifics. Likewise, in our study, we only observed one instance of potential triadic communication in the food begging context, with the video angle being insufficient to reliably code the signaller’s use of gaze alternation. Because of this relatively rare chance of observing interactions involving an external entity in naturally occurring communicative events, a more common way to study this in primates has been by using experimental food requesting paradigms, in which the subjects are confronted with an out of reach food item in the presence of a human experimenter (e.g. Leavens et al. 2004). Such studies revealed that ape (Bard 1992; Leavens and Hopkins 1998, Leavens et al. 2004; Lucca et al. 2018) but also some monkey species (e.g. squirrel monkeys: Anderson et al. 2010; olive baboons: Meunier et al. 2013; Tonkean macaques: Canteloup et al. 2015a) direct their (trained) begging gestures towards a food item in conjunction with showing gaze alternation between the experimenter and the food. Such observations are usually taken to suggest that the study subjects are capable of communicating about the food item to the experimenter in a (proto-) imperative manner (e.g. Bard 1992), i.e. with the aim of requesting the experimenter’s help to provide it to them. Although the debate about the psychological implications of such findings is still ongoing (e.g. Tomasello and Call 2019; Leavens 2021), from a philosophical point of view they imply that the signallers have an understanding of others as causal agents, i.e. they show first-order intentionality (Dennett 1983; Hurford 2007). A similar experimental setup was recently used in the same captive population of red-capped mangabeys as investigated in our study (Aychet et al. 2020). The study showed that the individuals of our study group were capable of this communicative capacity as well. In combination with the use of all the other behavioural markers of intentional communication during intraspecific communicative interactions (this study), the communication observed in this group of captive red-capped mangabeys is therefore remarkably similar to that found in the apes and can be classified as first-order intentional communication.
Flexibility of gesture use
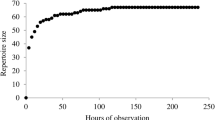
In terms of ‘means–ends dissociation’ (Bruner 1981), we found that the monkeys produced a quarter of their currently described gestural repertoire in more than one behavioural context. Furthermore, in both the social play and agonistic context the mangabeys produced several different gesture types (but see Pika and Deschner 2019 for a critical view on context assessment). These results suggest that the individuals studied here may use the same signal for different ends and different signals for the same end interchangeably, a capacity shared with the apes (e.g. Pika et al. 2005b; Liebal et al. 2006; Genty et al. 2009) as well as some other monkey species (pigtail macaques: Maestripieri 1996; Barbary macaques: Hesler and Fischer 2007; olive baboons: Molesti et al. 2020). It should be noted, however, that the proportion of gesture types used flexibly across contexts in our study group appears relatively low, i.e. not only when compared with the apes but also when compared with the other monkey species showing this form of communicative flexibility. There may be several explanations for this. One explanation is that the individual observation times in our study were probably too short to enable a complete understanding of the entire gestural repertoire and all its uses in our study population (see, e.g. Hobaiter and Byrne 2011). Indeed, a preliminary inspection of opportunistically recorded non-focal gesture use in the current study revealed that both the repertoire size and the proportion of gesture types used flexibly may be more extensive in this population than currently reported. Furthermore, differences in the observed degree of flexibility across study species and study populations may be due to the use of different definitions for gesture and different levels of detail used to describe gesture types and production contexts (e.g. Hobaiter and Byrne 2017; Molesti et al. 2020; Rodrigues et al. 2021). For instance, in the current study facial expressions were not considered gestures (see Byrne et al. 2017 for argumentation to classify facial expressions and gestures as independent systems), while in other monkey studies investigating intraspecific gesture use facial expressions were included in the analyses. Defining facial expressions as gestures inevitably leads to a larger repertoire size and, arguably, to a higher potential for flexible use. And another methodological issue to keep in mind is that differences in observed levels of flexibility across study groups may correspond to differences in group compositions and rearing environments (reviewed in Rodrigues et al. 2021). For example, when young individuals are over-represented in a group, higher levels of means–ends dissociation may be observed simply because young individuals tend to play more often than older individuals (e.g. Fröhlich et al. 2016), and play is characterised by a diversity of behavioural patterns from a variety of contexts (Yanagi and Berman 2014; Byrne 2016). From an ultimate perspective, an alternative explanation for the observed species differences in the level of means–ends dissociation could be that red-capped mangabeys have simply not developed a high degree of communicative flexibility in the visual domain. Although future studies with higher sample sizes, longer observation periods and uniform sampling methods are crucially needed to make valid species comparisons (Rodrigues et al. 2021), such a lack would be interesting, as it could reveal more about the selection pressures linked to the evolution of communicative complexity in relation to the specific socio-ecological environments that animals are reared in (e.g. ‘social complexity hypothesis for communication’: Freeberg et al. 2012; see also McComb and Semple 2005; Cartmill and Maestripieri 2012; Bard and Leavens 2014; Fröhlich and Hobaiter 2018; Peckre et al. 2019; Leavens et al. 2019; Rebout et al. 2020). Obtaining more insight into the differences and similarities of communicative capacities from a multitude of carefully chosen captive and wild representatives of the more than 50 genera of primates could therefore lead to a better understanding of the selection pressures that may have driven their origins, and, ultimately, the evolution of increasingly complex forms of intentional communication, including language (Leavens et al. 2019; Rodrigues et al. 2021).
Flexibility of goal persistence strategies
Another way of investigating flexibility of gesture use is by assessing a signallers’ use of goal persistence strategies in cases of communicative failure (Cartmill and Byrne 2007; 2010; Roberts et al. 2013). In the present study, we found a significant association between the type of further signalling behaviour by signallers and the type of unsatisfactory response given by their recipients. Persistence was the most frequent form of continuation when recipients did not respond with a behaviour change at all. Yet, in cases where recipients responded with an (apparently) ‘wrong’ behaviour change to the initial gesture, they did not show this preference for persistence, and often used elaboration as a form of further signalling behaviour as well. From a cognitive perspective, such a differentiation in communicative response characteristics to different forms of unsatisfactory responses from recipients could indicate that the red capped mangabey signallers, similarly to the apes (Cartmill and Byrne 2007; Leavens et al. 2005; Roberts et al. 2013; Genty et al. 2015), are capable of understanding different degrees of recipient comprehension, and use different types of repair strategies in response to this. In the ape studies, such findings are usually taken to suggest that the signallers are able to take into account the basic mental states of their recipients (Genty et al. 2015). Based on the results from the present study, in which the mangabeys behave very similar to apes in this specific domain of communicative complexity, the mangabeys might therefore be considered capable of basic mental state attribution as well. Notably, however, in a recent experimental study (Aychet et al. 2020) in which the same study population of red-capped mangabeys was subjected to an experimental design comparable to that generally used to assess a signaller’s sensitivity to recipient comprehension in ape studies, these monkeys showed a different response. In the study, the animals were trained to use begging gestures to request help from a human experimenter to access an out of reach food reward, with the experimenter showing different levels of responsiveness. The results from that study showed that the animals persisted using the same begging gesture in cases of receiving no response at all from the experimenter. Critically, however, they did not show signs of elaboration in cases of receiving the ‘wrong response’ from the experimenter. Although these results may reflect real interspecific differences with the apes, who have been found to show more flexibility in comparable experimental contexts (Aychet et al. 2020), the fact that in the current study we found more diverse response characteristics to apparent misunderstandings from recipients in intraspecific communicative interactions could indicate that these monkeys are actually more flexible in their communicative interactions than granted by experimental studies alone. In the experimental food begging context, the animals had been trained to use only one particular gesture type and may simply not have had an alternative ‘at hand’ when the communication failed in this novel context. These observed response differences across different study settings contribute to recent debates related to the question to what extent differences in testing conditions affect findings of gesture use across study populations (Bourjade et al. 2015; Leavens et al. 2019; Rodrigues et al. 2021). Moreover, they reiterate the importance of obtaining more data from both inter- and intraspecific communicative interactions from a more diverse range of species in both experimental and natural settings, to gain a better understanding of animals’ communicative capacities from a broader perspective (Rodrigues et al. 2021).
Manual gesture use
Finally, with regard to manual gesture production, we found that the monkeys made use of four ‘free’ manual gesture types. These were produced in variable frequencies divided over 11 of the 17 focal individuals. It has been suggested that the capacity for intentional and flexible manual gesture production formed the starting point of human language evolution, and, as manual gesture types were argued to be virtually non-existent in monkey species, that this starting point of human language evolution lies in the hominoid lineage (Call and Tomasello 2007; de Waal 2003, 2016; Pollick and de Waal 2007; de Waal and Pollick 2011; Cartmill and Maestripieri 2012; Roberts et al. 2012, 2014; Tomasello and Call 2019). The results from our study contradict this idea. The mere fact that the red capped mangabeys produced four free manual gesture types shows that intentionally produced manual gesture types are existent in monkey gestural repertoires. However, before further species comparisons can be made, systematic future studies assessing the proportion of manual gesture types in monkey species’ gesture repertoires, as well as their flexibility of use, are first needed. Based on our current findings, the hypothesis is that the proportion of manual gesture types in the gestural repertoire of red capped mangabeys will be comparable to that found in other monkey species living in similarly complex social societies (Hesler and Fischer 2007; Gupta and Sinha, 2019; Molesti et al. 2020), but will be considerably lower to that found in the apes (Genty et al. 2009; Hobaiter and Byrne 2011; Roberts et al. 2012, 2014; Graham et al. 2016; Knox et al. 2019). As mentioned, the assessment of the red capped mangabeys’ gestural repertoire is not complete yet and needs to be further investigated before further conclusions can be drawn about this.
Based on the results of our study, it currently seems that concerning flexibility and manual gesture use the apes outperform monkeys, although these capacities are not absent in monkey species investigated so far. Further comparative investigations of means–end dissociation, goal persistence and manual gesture use from a more diverse range of species are crucial to obtain a more complete view on these capacities in the primate lineage. This will reveal whether these capacities indeed turn out to be some of the key differences between monkey and ape communicative capacities (e.g. Cartmill and Maestripieri 2012), in addition to apes’ alleged capacities for higher order forms of intentionality underlying their communicative behaviour (e.g. Crockford et al. 2017) and turn-taking abilities (Pika et al. 2018). Yet, to unravel these differences it is important that uniform definitions and methodologies are used across studies. Investigating this further in future endeavours will shed more light on the presence of these capacities in other species than the apes, which can ultimately be used to better understand how human language evolved.
In sum, in this study, we provide systematic evidence that the captive population of red-capped mangabeys residing at the Station Biologique in Paimpoint use their naturally occurring body acts in flexible and intentional communicative ways to communicate with conspecifics. Specifically, their gestural production was characterised by all intentionality markers, thereby expanding previous studies that only tested subsets of markers of intentionality. The preliminary intentional gestural repertoire of our study population includes body postures, but also free movements of the hands and arms. They produce their gestures flexibly, both in terms of behaviour (e.g. elaboration during goal persistence) and context. In addition, they have been shown to engage in potential triadic gestural communication in an experimental food requesting paradigm (Aychet et al. 2020). In combination with the fact that studies on the vocal communication of these monkeys have indicated their capacity for syntactic like semantic vocal communication (Bouchet et al. 2010), we argue that red-capped mangabeys are a valuable species to consider for further comparative communication studies. The results of our study contradict claims that the starting point for flexible and intentional manual communicative strategies appeared in the hominoid lineage. In stark contrast, and in concordance with recent findings in bonnet macaques and olive baboons (Gupta and Sinha 2019, Molesti et al. 2020), our results show that some species belonging to the monkey lineage of catarrhine primates show these capacities as well. The difference between monkeys and apes may, therefore, be in degree, rather than in kind. Investigating which selection pressures have driven these differences in degree and have led to the highly complex forms of intentionality needed for ostensive communication found in human language are now needed to move the debate on human language origins to the next level.
Availability of data and materials
On request.
Code availability
On request.
References
Altmann J (1974) Observational study of behavior: sampling methods. Behaviour 49:227–267
Anderson JR, Kuroshima H, Hattori Y, Fujita K (2010) Flexibility in the use of requesting gestures in squirrel monkeys (Saimiri sciureus). Am J Primatol 72:707–714
Arbib MA, Liebal K, Pika S (2008) Primate vocalization, gesture, and the evolution of human language. Curr Anthropol 49:1053–1076
Aychet J, Pezzino P, Rossard A, Bec P, Blois-Heulin C, Lemasson A (2020) Red-capped mangabeys (Cercocebus torquatus) adapt their interspecific gestural communication to the recipient’s behaviour. Sci Rep 10(1):1–12
Bard K (1992) Intentional behaviour and intentional communication in young free-ranging orangutans. Child Dev 63:1186–1197
Bard KA, Leavens DA (2014) The importance of development for comparative primatology. Annu Rev Anthropol 43:83–200
Bard KA, Dunbar S, Maguire-Herring V, Veira Y, Hayes KG, McDonald K (2014) Gestures and social-emotional communicative development in chimpanzee infants. Am J Primatol 76(1):14–29
Bates E, Camaioni L, Volterra V (1975) The acquisition of performatives prior to speech. Merrill-Palmer Q Behav Dev 21(3):205–226
Ben Mocha Y, Burkart JM (2021) Intentional communication: solving methodological issues to assigning first-order intentional signalling. Biol Rev 96(3):903–921
Bouchet H, Pellier AS, Blois-Heulin C, Lemasson A (2010) Sex differences in the vocal repertoire of adult red-capped mangabeys (Cercocebus torquatus): a multi-level acoustic analysis. Am J Primatol 72(4):360–375
Bouchet H, Blois-Heulin C, Lemasson A (2013) Social complexity parallels vocal complexity: a comparison of three non-human primate species. Front Psychol 4:390
Bourjade M, Meguerditchian A, Maille A, Gaunet F, Vauclair J (2014) Olive baboons, Papio anubis, adjust their visual and auditory intentional gestures to the visual attention of others. Anim Behav 87:121–128
Bourjade M, Canteloup C, Meguerditchian A, Vauclair J, Gaunet F (2015) Training experience in gestures affects the display of social gaze in baboons’ communication with a human. Anim Cogn 18(1):239–250
Bruner J (1981) Intention in the structure of action and interaction. In: Lipsett L (ed) Advances in infancy research. Ablex, Norwood
Byrne RW (2016) Evolving insight. Oxford University Press
Byrne RW, Cartmill E, Genty E, Graham KE, Hobaiter C, Tanner J (2017) Great ape gestures: intentional communication with a rich set of innate signals. Anim Cogn 20(4):755–769
Call J, Tomasello M (1994) Production and comprehension of referential pointing by orangutans (Pongo pygmaeus). J Comp Psychol 108(4):307
Call J, Tomasello M (eds) (2007) The gestural communication of apes and monkeys. Lawrence Erlbaum, Mahwah, London
Canteloup C, Bovet D, Meunier H (2015a) Do Tonkean macaques (Macaca tonkeana) tailor their gestural and visual signals to fit the attentional states of a human partner? Anim Cogn 18:451–461
Canteloup C, Bovet D, Meunier H (2015b) Intentional gestural communication and discrimination of human attentional states in rhesus macaques (Macaca mulatta). Anim Cogn 18:875–883
Cartmill EA, Byrne RW (2007) Orangutans modify their gestural signaling according to their audience’s comprehension. Curr Biol 17:1345–1348
Cartmill EA, Byrne RW (2010) Semantics of primate gestures: intentional meanings of orangutan gestures. Anim Cogn 13:793–804
Cartmill EA, Hobaiter C (2019) Gesturing towards the future: cognition, big data, and the future of comparative gesture research. Anim Cogn 22(4):597–604
Cartmill EA, Maestripieri D (2012) Socio-cognitive specializations in non-human primates: evidence from gestural communication. The Oxford handbook of comparative evolutionary psychology. Oxford University Press, Oxford, pp 166–193
Chalmers NR (1968) Group composition, ecology and daily activities of free living mangabeys in Uganda. Folia Primatol (basel) 8:247–262
Cooke CA (2012) The feeding, ranging and positional behaviors of Cercocebus torquatus, the red-capped mangabey, in Sette Cama Gabon: a phylogenetic perspective. Doctoral dissertation, Ohio State University
Crockford C, Wittig RM, Zuberbühler K (2017) Vocalizing in chimpanzees is influenced by social-cognitive processes. Sci Adv 3(11):e1701742
de Waal F (2003) Darwin’s legacy and the study of primate visual communication. Ann N Y Acad Sci 1000:7–31
de Waal F (2016) Are we smart enough to know how smart animals are? WW Norton and Company
de Waal FB, Pollick AS (2011) Gesture as the most flexible modality of primate communication. In: The Oxford handbook of language evolution
Defolie C, Malassis R, Serre M, Meunier H (2015) Tufted capuchins (Cebus apella) adapt their communicative behaviour to human’s attentional states. Anim Cogn 18:747–755
Dennett DC (1983) Intentional systems in cognitive ethology: The “Panglossian paradigm” defended. Behav Brain Sci 6(3):343–390
Deputte B (1992) Life history of captive gray-cheeked mangabeys: physical and sexual development. Int J Primatol 13:509–531
Dolado R, Beltran FS (2012) Emergent patterns of social organization in captive Cercocebus torquatus: testing the GrooFiWorld agent-based model. J Biosci 37(4):777–784
Fischer J, Price T (2016). Meaning, intention, and inference in primate vocal communication. Neurosci Biobehav Rev
Fitch WT (2010) The evolution of language. Cambridge University Press, Cambridge
Fitch WT (2016) Why formal semantics and primate communication make strange bedfellows. Theor Linguist 42:97–109
Fitch WT (2017) Empirical approaches to the study of language evolution. Psychon Bull Rev 24:3–33
Freeberg TM, Dunbar RI, Ord TJ (2012) Social complexity as a proximate and ultimate factor in communicative complexity. Phil Trans R Soc B 367:1785–1801
Fröhlich M, Hobaiter C (2018) The development of gestural communication in great apes. Behav Ecol Sociobiol 72(12):194
Fröhlich M, Wittig RM, Pika S (2016) Play-solicitation gestures in chimpanzees in the wild: flexible adjustment to social circumstances and individual matrices. R Soc Open Sci 3(8):160278
Gautier-Hion A, Gautier JP (1976) Croissance, maturité sexuelle et sociale, reproduction chez les cercopithécinés forestiers africains. Folia Primatol 26(3):165–184
Gautier-Hion A, Colyn M, Gautier JP (1999). Histoire naturelle des primates d’Afrique centrale. ECOFAC, Gabon
Genty E, Breuer T, Hobaiter C, Byrne RW (2009) Gestural communication of the gorilla (Gorilla gorilla): repertoire, intentionality and possible origins. Anim Cogn 12:527–546
Genty E, Neumann C, Zuberbühler K (2015) Bonobos modify communication signals according to recipient familiarity. Sci Rep 5(1):1–10
Graham KE, Hobaiter C, Byrne RW (2016) Expressed and understood gestural repertoires of wild bonobos (Pan paniscus). PeerJ Preprints
Graham KE, Furuichi T, Byrne RW (2017) The gestural repertoire of the wild bonobo (Pan paniscus): a mutually understood communication system. Anim Cogn 20:171–177. https://doi.org/10.1007/s10071-016-1035-9
Graham KE, Wilke C, Lahiff NJ, Slocombe KE (2020) Scratching beneath the surface: intentionality in great ape signal production. Philos Trans R Soc B 375(1789):20180403
Grice P (1957) Meaning. Philos Rev 66:377–388
Gupta S, Sinha A (2019) Gestural communication of wild bonnet macaques in the Bandipur National Park Southern India. Behav Process 168:103956
Hattori Y, Kuroshima H, Fujita K (2007) I know you are not looking at me: capuchin monkeys’ (Cebus apella) sensitivity to human attentional states. Anim Cogn 10(2):141
Hattori Y, Kuroshima H, Fujita K (2010) Tufted capuchin monkeys (Cebus apella) show understanding of human attentional states when requesting food held by a human. Anim Cogn 13:87–92
Hauser MD, Chomsky N, Fitch WT (2002) The faculty of language: what is it, who has it, and how did it evolve? Science 298:1569–1579
Hesler N, Fischer J (2007) Gestural communication in Barbary macaques (Macaca sylvanus): an overview. In: Call J, Tomasello M (eds) The gestural communication of apes and monkeys. Lawrence Erlbaum, Mahwah and London
Hewes GW, Andrew RJ, Carini L, Choe H, Gardner RA, Kortlandt A, Krantz GS, McBride G, Nottebohm F, Pfeiffer J, Rumbaugh DG (1973) Primate communication and the gestural origin of language [and comments and reply]. Curr Anthropol 14(1/2):5–24
Hobaiter C, Byrne RW (2011) The gestural repertoire of the wild chimpanzee. Anim Cogn 14:745–767
Hobaiter C, Byrne RW (2014) The meanings of chimpanzee gestures. Curr Biol 24(14):1596–1600
Hobaiter C, Byrne RW (2017) What is a gesture? A meaning-based approach to defining gestural repertoires. Neurosci Biobehav Rev 82:3–12
Hockett CF (1959) Animal “languages” and human language. Hum Biol 31:32–39
Hostetter AB, Cantero M, Hopkins WD (2001) Differential use of vocal and gestural communication by chimpanzees (Pan troglodytes) in response to the attentional status of a human (Homo sapiens). J Comp Psychol 115:337–343
Hurford JR (2007) Language in the light of evolution: vol 1, the origins of meaning. Oxford University Press, Oxford
Jones C, Sabater Pi J (1968) Comparative ecology of Cercocebus albigena (Gray) and Cercocebus torquatus (Kerr) in Rio Muni, West Africa. Folia Primatol 9:99–113
Knox A, Markx J, How E, Azis A, Hobaiter C, van Veen FJ, Morrogh-Bernard H (2019) Gesture use in communication between mothers and offspring in wild orang-utans (Pongo pygmaeus wurmbii) from the Sabangau Peat-Swamp Forest Borneo. Int J Primatol 40(3):393–416
Krams I, Krama T, Freeberg TM, Kullberg C, Lucas JR (2012) Linking social complexity and vocal complexity: a parid perspective. Phil Trans R Soc B 367:1879–1891
Leavens DA (2021) The Referential Problem Space revisited: An ecological hypothesis of the evolutionary and developmental origins of pointing. Wiley Interdiscip Rev Cogn Sci 12(4):e1554
Leavens DA, Hopkins WD (1998) Intentional communication by chimpanzees: a cross-sectional study of the use of referential gestures. Dev Psych 34:813–822
Leavens DA, Hopkins WD, Bard KA (1996) Indexical and referential pointing in chimpanzees (Pan troglodytes). J Comp Psychol 110(4):346
Leavens DA, Hostetter AB, Wesley MJ, Hopkins WD (2004) Tactical use of unimodal and bimodal communication by chimpanzees (Pan troglodytes). Anim Behav 67:467–476
Leavens DA, Russell JL, Hopkins WD (2005) Intentionality as measured in the persistence and elaboration of communication by chimpanzees (Pan troglodytes). Child Dev 76:291–306
Leavens DA, Bard KA, Hopkins WD (2019) The mismeasure of ape social cognition. Anim Cogn 22(4):487–504
Lemasson A, Ouattara K, Zuberbühler K (2013) Exploring the gaps between primate calls and human language. Evol Emerg Lang Evid Inference 17:181
Liebal K, Call J (2012) The origins of non-human primates’ manual gestures. Philos Trans R Soc b: Biol Sci 367(1585):118–128
Liebal K, Pika S, Tomasello M (2004) Social communication in siamangs (Symphalangus syndactylus): use of gestures and facial expressions. Primates 45:41–57
Liebal K, Pika S, Tomasello M (2006) Gestural communication of orangutans (Pongo pygmaeus). Gesture 6:1–38
Liebal K, Waller B, Burrows A, Slocombe K (2013) Primate communication: a multimodal approach. Cambridge University Press, Cambridge
Lucca K, MacLean E, Hare B (2018) The development and flexibility of gaze alternations in bonobos and chimpanzees. Dev Sci 21(4):e12598
Maestripieri D (1996) Gestural communication and its cognitive implications in pigtail macaques (Macaca nemestrina). Behaviour 133(13–14):997–1022
Maestripieri D (1999) Primate social organization, gestural repertoire size, and communication dynamics: a comparative study of macaques. In: King BJ (ed) The origins of language. What nonhuman primates can tell us. School of American Research, Santa Fe, pp 55–77
Maille A, Engelhart L, Bourjade M, Blois-Heulin C (2012) To beg, or not to beg? That is the question: mangabeys modify their production of requesting gestures in response to human’s attentional states. PLoS ONE 7(7):e41197
Martin DO, Austin H (1996) Exact estimates for a rate ratio. Epidemiology 7:29–33
McComb K, Semple S (2005) Coevolution of vocal communication and sociality in primates. Biol Let 1:381–385
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochemia Medica: Biochemia Medica 22(3):276–282
Meunier H, Prieur J, Vauclair J (2013) Olive baboons communicate intentionally by pointing. Anim Cogn 16:155–163
Molesti S, Meguerditchian A, Bourjade M (2020) Gestural communication in olive baboons (Papio anubis): repertoire and intentionality. Anim Cogn 23(1):19–40
Moore R (2016) Meaning and ostension in great-ape gestural communication. Anim Cogn 19:223–231
Orimaye OJ (2017) Density and abundance of the red-capped mangabey (Cercocebus torquatus) in omo biosphere reserve and idanre forest reserve south western Nigeria. MOJ Proteomics Bioinforma. https://doi.org/10.15406/mojpb.2017.05.00156
Peckre L, Kappeler PM, Fichtel C (2019) Clarifying and expanding the social complexity hypothesis for communicative complexity. Behav Ecol Sociobiol 73(1):11
Petkov CI, Jarvis ED (2012) Birds, primates, and spoken language origins: behavioral phenotypes and neurobiological substrates. Front Evol Neurosci 4
Pika S (2008) What is the nature of the gestural communication of great apes. The shared mind 165-86
Pika S (2017) Unpeeling the layers of communicative complexity. Anim Behav 134:223–227
Pika S, Deschner T (2019) A new window onto animal culture: The case of chimpanzee gesturing. Gesture 18(2–3):239–260
Pika S, Liebal K (2012) Developments in primate gesture research. John Benjamins Publishing
Pika S, Liebal K, Tomasello M (2003) Gestural communication in young gorillas (Gorilla gorilla): gestural repertoire, learning, and use. Am J Primatol 60:95–111
Pika S, Liebal K, Call J, Tomasello M (2005a) Gestural communication of apes. Gesture 5(1–2):41–56
Pika S, Liebal K, Tomasello M (2005b) Gestural communication in subadult bonobos (Pan paniscus): repertoire and use. Am J Primatol 65(1):39–61
Pika S, Wilkinson R, Kendrick KH, Vernes SC (2018) Taking turns: bridging the gap between human and animal communication. Proc R Soc B 285(1880):20180598
Plooij FX (1978) Some basic traits of language in wild chimpanzees? In: Lock A (ed) Action, gesture and symbol: the emergence of language. Academic Press, London, pp 111–131
Plooij FX (1984) The behavioral development of free-living chimpanzee babies and infants. Monographs on infancy
Pollard KA, Blumstein DT (2012) Evolving communicative complexity: insights from rodents and beyond. Philos Trans R Soc b: Biol Sci 367(1597):1869–1878
Pollick AS, De Waal FB (2007) Ape gestures and language evolution. Proc Natl Acad Sci 104:8184–8189
Premack D, Woodruff G (1978) Does the chimpanzee have a theory of mind? Behav Brain Sci 1(4):515–526
Rebout N, De Marco A, Lone JC, Sanna A, Cozzolino R, Micheletta J, Sterck EHM, Langermans JA, Lemasson A, Thierry B (2020) Tolerant and intolerant macaques show different levels of structural complexity in their vocal communication. Proc R Soc B 287(1928):20200439
Roberts AI, Roberts SGB (2019). Social bonding, gestural complexity and displacement behaviour of wild chimpanzee. bioRxiv, p 678805
Roberts AI, Vick SJ, Roberts SGB, Buchanan-Smith HM, Zuberbühler K (2012) A structure-based repertoire of manual gestures in wild chimpanzees: statistical analyses of a graded communication system. Evol Hum Behav 33:578–589
Roberts AI, Vick SJ, Buchanan-Smith HM (2013) Communicative intentions in wild chimpanzees: persistence and elaboration in gestural signalling. Anim Cogn 16(2):187–196
Roberts AI, Roberts SG, Vick SJ (2014) The repertoire and intentionality of gestural communication in wild chimpanzees. Anim Cogn 17:317–336
Roberts AI, Murray L, Roberts SGB (2019) Complex sociality of wild chimpanzees can emerge from laterality of manual gestures. Hum Nat 30(3):299–325
Rodrigues ED, Santos AJ, Veppo F, Pereira J, Hobaiter C (2021) Connecting primate gesture to the evolutionary roots of language: a systematic review. Am J Primatol 83(9):e23313
Sahai H, Khurshid A (1996) Formulae and tables for the determination of sample sizes and power in clinical trials for testing differences in proportions for the two-sample design: a review. Stat Med 15(1):1–21
Schel AM, Townsend SW, Machanda Z, Zuberbühler K, Slocombe KE (2013) Chimpanzee alarm call production meets key criteria for intentionality. PLoS ONE 8(10):e76674
Scott-Phillips TC (2015) Meaning in animal and human communication. Anim Cogn 18:801–805
Searle JR, Willis S (1983) Intentionality: an essay in the philosophy of mind. Cambridge University Press, Cambridge
Seyfarth RM (2005) Continuities in vocal communication argue against a gestural origin of language. Behav Brain Sci 28(2):144
Snowdon CT, Brown CH, Petersen MR (1982) Primate communication. Cambridge University Press, Cambridge
Sperber D, Wilson D (1995) Relevance: communication and cognition. Blackwell, Oxford
Tomasello M (2010) Origins of human communication. MIT Press
Tomasello M, Call J (2019) Thirty years of great ape gestures. Anim Cogn 22(4):461–469
Tomasello M, George BL, Kruger AC, Farrar MJ, Evans A (1985) The development of gestural communication in young chimpanzees. J Hum Evol 14:175–186
Tomasello M, Call J, Nagell K, Olguin R, Carpenter M (1994) The learning and the use of gestural signals by young chimpanzees: a trans-generational study. Primates 35:137–154
Tomasello M, Call J, Warren J, Frost GT, Carpenter M, Nagell K (1997) The ontogeny of chimpanzee gestural signals: a comparison across groups and generations. Evol Commun 1(2):223–259
Townsend SW, Koski SE, Byrne RW, Slocombe KE, Bickel B, Boeckle M, Braga Goncalves I, Burkart JM, Flower T, Gaunet F, Glock HJ et al (2017) Exorcising Grice’s ghost: an empirical approach to studying intentional communication in animals. Biol Rev 92:1427–1433
Yanagi A, Berman CM (2014) Body signals during social play in free-ranging rhesus macaques (Macaca mulatta): a systematic analysis. Am J Primatol 76:168–179
Funding
This study was funded by Fondation Fyssen to AS, the Dr. J. L. Dobberke Stichting voor Vergelijkende Psychologie of the Koninklijke Academie van Wetenschappen to AS, and a grant of the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (Grant agreement no. 772000) to SP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Schel, A.M., Bono, A., Aychet, J. et al. Intentional gestural communication amongst red-capped mangabeys (Cercocebus torquatus). Anim Cogn 25, 1313–1330 (2022). https://doi.org/10.1007/s10071-022-01615-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-022-01615-7