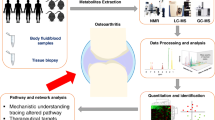
Abstract
Osteoarthritis (OA) represents the most prevalent and disabling arthritis worldwide due to its heterogeneous and progressive articular degradation. However, effective and timely diagnosis and fundamental treatment for this disorder are lacking. Metabolomics, a growing field in life science research in recent years, has the potential to detect many metabolites and thus explains the underlying pathophysiological processes. Hence, new specific metabolic markers and related metabolic pathways can be identified for OA. In this review, we aimed to provide an overview of studies related to the metabolomics of OA in animal models and humans to describe the metabolic changes and related pathways for OA. The present metabolomics studies reveal that the pathogenesis of OA may be significantly related to perturbations of amino acid metabolism. These altered amino acids (e.g., branched-chain amino acids, arginine, and alanine), as well as phospholipids, were identified as potential biomarkers to distinguish patients with OA from healthy individuals.





Similar content being viewed by others
Abbreviations
- ACLT:
-
anterior cruciate ligament transaction
- ARG:
-
arginase
- ATP:
-
adenosine triphosphate
- BCAA:
-
branched-chain amino acids
- GC–MS:
-
gas chromatography–mass spectrometry
- LC–MS:
-
liquid chromatography–mass spectrometry
- LysoPC:
-
lysophosphatidylcholine
- LPA:
-
lysophosphatidic acid
- MD:
-
meniscal destabilization
- mTOR:
-
mammalian target of rapamycin
- NMR:
-
nuclear magnetic resonance
- NO:
-
nitric oxide
- NOS:
-
NO synthase
- OA:
-
osteoarthritis
- OAT:
-
ornithine aminotransferase
- PC:
-
phosphatidylcholine
- PLA2:
-
phospholipase A2
- SF:
-
synovial fluid
- TCA:
-
tricarboxylic acid
- UPLC-MS:
-
ultra-performance liquid chromatography-tandem mass spectrometry
References
Woolf AD, Pfleger B (2003) Burden of major musculoskeletal conditions. Bull World Health Organ 81(9):646–656
Martel-Pelletier J, Barr AJ, Cicuttini FM, Conaghan PG, Cooper C, Goldring MB, Goldring SR, Jones G, Teichtahl AJ, Pelletier JP (2016) Osteoarthritis. Nature reviews Disease primers 2:16072. https://doi.org/10.1038/nrdp.2016.72
Loeser RF, Goldring SR, Scanzello CR, Goldring MB (2012) Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum 64(6):1697–1707. https://doi.org/10.1002/art.34453
Hiligsmann M, Cooper C, Arden N, Boers M, Branco JC, Luisa Brandi M, Bruyère O, Guillemin F, Hochberg MC, Hunter DJ, Kanis JA, Kvien TK, Laslop A, Pelletier JP, Pinto D, Reiter-Niesert S, Rizzoli R, Rovati LC, Severens JL, Silverman S, Tsouderos Y, Tugwell P, Reginster JY (2013) Health economics in the field of osteoarthritis: an expert’s consensus paper from the European Society for Clinical and Economic Aspects of osteoporosis and osteoarthritis (ESCEO). Semin Arthritis Rheum 43(3):303–313. https://doi.org/10.1016/j.semarthrit.2013.07.003
Zhai G (2019) Alteration of metabolic pathways in osteoarthritis. Metabolites 9(1). https://doi.org/10.3390/metabo9010011
Nepple JJ, Thomason KM, An TW, Harris-Hayes M, Clohisy JC (2015) What is the utility of biomarkers for assessing the pathophysiology of hip osteoarthritis? A systematic review. Clin Orthop Relat Res 473(5):1683–1701. https://doi.org/10.1007/s11999-015-4148-6
Showiheen SAA, Sun AR, Wu X, Crawford R, Xiao Y, Wellard RM, Prasadam I (2019) Application of metabolomics to osteoarthritis: from basic science to the clinical approach. Curr Rheumatol Rep 21(6):26. https://doi.org/10.1007/s11926-019-0827-8
Nicholson JK, Lindon JC (2008) Systems biology: metabonomics. Nature 455(7216):1054–1056. https://doi.org/10.1038/4551054a
Priori R, Scrivo R, Brandt J, Valerio M, Casadei L, Valesini G, Manetti C (2013) Metabolomics in rheumatic diseases: the potential of an emerging methodology for improved patient diagnosis, prognosis, and treatment efficacy. Autoimmun Rev 12(10):1022–1030. https://doi.org/10.1016/j.autrev.2013.04.002
Cui L, Zheng D, Lee YH, Chan TK, Kumar Y, Ho WE, Chen JZ, Tannenbaum SR, Ong CN (2016) Metabolomics investigation reveals metabolite mediators associated with acute lung injury and repair in a murine model of influenza pneumonia. Sci Rep 6:26076. https://doi.org/10.1038/srep26076
Weljie AM, Newton J, Mercier P, Carlson E, Slupsky CM (2006) Targeted profiling: quantitative analysis of 1H NMR metabolomics data. Anal Chem 78(13):4430–4442. https://doi.org/10.1021/ac060209g
Johnson CH, Ivanisevic J, Siuzdak G (2016) Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol 17(7):451–459. https://doi.org/10.1038/nrm.2016.25
Gupta L, Ahmed S, Jain A, Misra R (2018) Emerging role of metabolomics in rheumatology. Int J Rheum Dis 21(8):1468–1477. https://doi.org/10.1111/1756-185x.13353
Halama A (2014) Metabolomics in cell culture--a strategy to study crucial metabolic pathways in cancer development and the response to treatment. Arch Biochem Biophys 564:100–109. https://doi.org/10.1016/j.abb.2014.09.002
McGarrah RW, Crown SB, Zhang GF, Shah SH, Newgard CB (2018) Cardiovascular metabolomics. Circ Res 122(9):1238–1258. https://doi.org/10.1161/circresaha.117.311002
Tavares G, Venturini G, Padilha K, Zatz R, Pereira AC, Thadhani RI, Rhee EP, Titan SMO (2018) 1,5-Anhydroglucitol predicts CKD progression in macroalbuminuric diabetic kidney disease: results from non-targeted metabolomics. Metabolomics: official Journal of the Metabolomic Society 14(4):39. https://doi.org/10.1007/s11306-018-1337-9
Weckmann K, Diefenthäler P, Baeken MW, Yusifli K, Turck CW, Asara JM, Behl C, Hajieva P (2018) Metabolomics profiling reveals differential adaptation of major energy metabolism pathways associated with autophagy upon oxygen and glucose reduction. Sci Rep 8(1):2337. https://doi.org/10.1038/s41598-018-19421-y
Zhang A, Sun H, Yan G, Wang P, Han Y, Wang X (2014) Metabolomics in diagnosis and biomarker discovery of colorectal cancer. Cancer Lett 345(1):17–20. https://doi.org/10.1016/j.canlet.2013.11.011
Jacob M, Lopata AL, Dasouki M, Abdel Rahman AM (2019) Metabolomics toward personalized medicine. Mass Spectrom Rev 38(3):221–238. https://doi.org/10.1002/mas.21548
Markley JL, Brüschweiler R, Edison AS, Eghbalnia HR, Powers R, Raftery D, Wishart DS (2017) The future of NMR-based metabolomics. Curr Opin Biotechnol 43:34–40. https://doi.org/10.1016/j.copbio.2016.08.001
Heather LC, Wang X, West JA, Griffin JL (2013) A practical guide to metabolomic profiling as a discovery tool for human heart disease. J Mol Cell Cardiol 55:2–11. https://doi.org/10.1016/j.yjmcc.2012.12.001
Kobayashi T, Yoshihara Y, Yamada H, Fujikawa K (2000) Procollagen IIC-peptide as a marker for assessing mechanical risk factors of knee osteoarthritis: effect of obesity and varus alignment. Ann Rheum Dis 59(12):982–984. https://doi.org/10.1136/ard.59.12.982
Dettmer K, Aronov PA, Hammock BD (2007) Mass spectrometry-based metabolomics. Mass Spectrom Rev 26(1):51–78. https://doi.org/10.1002/mas.20108
Blair-Levy JM, Watts CE, Fiorentino NM, Dimitriadis EK, Marini JC, Lipsky PE (2008) A type I collagen defect leads to rapidly progressive osteoarthritis in a mouse model. Arthritis Rheum 58(4):1096–1106. https://doi.org/10.1002/art.23277
Hui AY, McCarty WJ, Masuda K, Firestein GS, Sah RL (2012) A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley interdisciplinary reviews Systems biology and medicine 4(1):15–37. https://doi.org/10.1002/wsbm.157
Whitelaw CB, Sheets TP, Lillico SG, Telugu BP (2016) Engineering large animal models of human disease. J Pathol 238(2):247–256. https://doi.org/10.1002/path.4648
Carlson AK, Rawle RA, Wallace CW, Brooks EG, Adams E, Greenwood MC, Olmer M, Lotz MK, Bothner B, June RK (2019) Characterization of synovial fluid metabolomic phenotypes of cartilage morphological changes associated with osteoarthritis. Osteoarthr Cartil 27(8):1174–1184. https://doi.org/10.1016/j.joca.2019.04.007
Williamson MP, Humm G, Crisp AJ (1989) 1H nuclear magnetic resonance investigation of synovial fluid components in osteoarthritis, rheumatoid arthritis and traumatic effusions. Br J Rheumatol 28(1):23–27. https://doi.org/10.1093/rheumatology/28.1.23
Holmes E, Wilson ID, Nicholson JK (2008) Metabolic phenotyping in health and disease. Cell 134(5):714–717. https://doi.org/10.1016/j.cell.2008.08.026
Shet K, Siddiqui SM, Yoshihara H, Kurhanewicz J, Ries M, Li X (2012) High-resolution magic angle spinning NMR spectroscopy of human osteoarthritic cartilage. NMR Biomed 25(4):538–544. https://doi.org/10.1002/nbm.1769
Adams SB Jr, Setton LA, Kensicki E, Bolognesi MP, Toth AP, Nettles DL (2012) Global metabolic profiling of human osteoarthritic synovium. Osteoarthr Cartil 20(1):64–67. https://doi.org/10.1016/j.joca.2011.10.010
Yang G, Zhang H, Chen T, Zhu W, Ding S, Xu K, Xu Z, Guo Y, Zhang J (2016) Metabolic analysis of osteoarthritis subchondral bone based on UPLC/Q-TOF-MS. Anal Bioanal Chem 408(16):4275–4286. https://doi.org/10.1007/s00216-016-9524-x
Nguyen LT, Sharma AR, Chakraborty C, Saibaba B, Ahn ME, Lee SS (2017) Review of prospects of biological fluid biomarkers in osteoarthritis. Int J Mol Sci 18(3). https://doi.org/10.3390/ijms18030601
Maher AD, Coles C, White J, Bateman JF, Fuller ES, Burkhardt D, Little CB, Cake M, Read R, McDonagh MB, Rochfort SJ (2012) 1H NMR spectroscopy of serum reveals unique metabolic fingerprints associated with subtypes of surgically induced osteoarthritis in sheep. J Proteome Res 11(8):4261–4268. https://doi.org/10.1021/pr300368h
Costello CA, Hu T, Liu M, Zhang W, Furey A, Fan Z, Rahman P, Randell EW, Zhai G (2019) Metabolomics signature for non-responders to total joint replacement surgery in primary osteoarthritis patients: the Newfoundland Osteoarthritis Study. Journal of orthopaedic research: official publication of the Orthopaedic Research Society. doi:https://doi.org/10.1002/jor.24529
Zhang W, Sun G, Likhodii S, Liu M, Aref-Eshghi E, Harper PE, Martin G, Furey A, Green R, Randell E, Rahman P, Zhai G (2016) Metabolomic analysis of human plasma reveals that arginine is depleted in knee osteoarthritis patients. Osteoarthr Cartil 24(5):827–834. https://doi.org/10.1016/j.joca.2015.12.004
Zhai G, Wang-Sattler R, Hart DJ, Arden NK, Hakim AJ, Illig T, Spector TD (2010) Serum branched-chain amino acid to histidine ratio: a novel metabolomic biomarker of knee osteoarthritis. Ann Rheum Dis 69(6):1227–1231. https://doi.org/10.1136/ard.2009.120857
Ryan D, Robards K, Prenzler PD, Kendall M (2011) Recent and potential developments in the analysis of urine: a review. Anal Chim Acta 684(1–2):8–20. https://doi.org/10.1016/j.aca.2010.10.035
Loeser RF, Pathmasiri W, Sumner SJ, McRitchie S, Beavers D, Saxena P, Nicklas BJ, Jordan J, Guermazi A, Hunter DJ, Messier SP (2016) Association of urinary metabolites with radiographic progression of knee osteoarthritis in overweight and obese adults: an exploratory study. Osteoarthr Cartil 24(8):1479–1486. https://doi.org/10.1016/j.joca.2016.03.011
Messier SP, Mihalko SL, Legault C, Miller GD, Nicklas BJ, DeVita P, Beavers DP, Hunter DJ, Lyles MF, Eckstein F, Williamson JD, Carr JJ, Guermazi A, Loeser RF (2013) Effects of intensive diet and exercise on knee joint loads, inflammation, and clinical outcomes among overweight and obese adults with knee osteoarthritis: the IDEA randomized clinical trial. Jama 310(12):1263–1273. https://doi.org/10.1001/jama.2013.277669
Li X, Yang SB, Qiu YP, Zhao T, Chen TL, Su MM, Chu LX, Lv AP, Liu P, Jia W (2010) Urinary metabolomics as a potentially novel diagnostic and stratification tool for knee osteoarthritis. Metabolomics: official journal of the Metabolomic Society 6(1):109–118. https://doi.org/10.1007/s11306-009-0184-0
Blanco FJ (2014) Osteoarthritis year in review 2014: we need more biochemical biomarkers in qualification phase. Osteoarthr Cartil 22(12):2025–2032. https://doi.org/10.1016/j.joca.2014.09.009
Carlson AK, Rawle RA, Adams E, Greenwood MC, Bothner B, June RK (2018) Application of global metabolomic profiling of synovial fluid for osteoarthritis biomarkers. Biochem Biophys Res Commun 499(2):182–188. https://doi.org/10.1016/j.bbrc.2018.03.117
Mickiewicz B, Kelly JJ, Ludwig TE, Weljie AM, Wiley JP, Schmidt TA, Vogel HJ (2015) Metabolic analysis of knee synovial fluid as a potential diagnostic approach for osteoarthritis. Journal of orthopaedic research: official publication of the Orthopaedic Research Society 33(11):1631–1638. https://doi.org/10.1002/jor.22949
Mickiewicz B, Heard BJ, Chau JK, Chung M, Hart DA, Shrive NG, Frank CB, Vogel HJ (2015) Metabolic profiling of synovial fluid in a unilateral ovine model of anterior cruciate ligament reconstruction of the knee suggests biomarkers for early osteoarthritis. Journal of orthopaedic research: official publication of the Orthopaedic Research Society 33(1):71–77. https://doi.org/10.1002/jor.22743
Zhang W, Sun G, Aitken D, Likhodii S, Liu M, Martin G, Furey A, Randell E, Rahman P, Jones G, Zhai G (2016) Lysophosphatidylcholines to phosphatidylcholines ratio predicts advanced knee osteoarthritis. Rheumatology (Oxford, England) 55(9):1566–1574. https://doi.org/10.1093/rheumatology/kew207
Borel M, Pastoureau P, Papon J, Madelmont JC, Moins N, Maublant J, Miot-Noirault E (2009) Longitudinal profiling of articular cartilage degradation in osteoarthritis by high-resolution magic angle spinning 1H NMR spectroscopy: experimental study in the meniscectomized Guinea pig model. J Proteome Res 8(5):2594–2600. https://doi.org/10.1021/pr8009963
Senol O, Gundogdu G, Gundogdu K, Miloglu FD (2019) Investigation of the relationships between knee osteoarthritis and obesity via untargeted metabolomics analysis. Clin Rheumatol 38(5):1351–1360. https://doi.org/10.1007/s10067-019-04428-1
Zhenyukh O, Civantos E, Ruiz-Ortega M, Sánchez MS, Vázquez C, Peiró C, Egido J, Mas S (2017) High concentration of branched-chain amino acids promotes oxidative stress, inflammation and migration of human peripheral blood mononuclear cells via mTORC1 activation. Free Radic Biol Med 104:165–177. https://doi.org/10.1016/j.freeradbiomed.2017.01.009
Kluzek S, Newton JL, Arden NK (2015) Is osteoarthritis a metabolic disorder? Br Med Bull 115(1):111–121. https://doi.org/10.1093/bmb/ldv028
Lynch CJ, Adams SH (2014) Branched-chain amino acids in metabolic signalling and insulin resistance. Nat Rev Endocrinol 10(12):723–736. https://doi.org/10.1038/nrendo.2014.171
Bonvini A, Coqueiro AY, Tirapegui J, Calder PC, Rogero MM (2018) Immunomodulatory role of branched-chain amino acids. Nutr Rev 76(11):840–856. https://doi.org/10.1093/nutrit/nuy037
Bassit RA, Sawada LA, Bacurau RF, Navarro F, Costa Rosa LF (2000) The effect of BCAA supplementation upon the immune response of triathletes. Med Sci Sports Exerc 32(7):1214–1219. https://doi.org/10.1097/00005768-200007000-00005
Kapoor M, Martel-Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H (2011) Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 7(1):33–42. https://doi.org/10.1038/nrrheum.2010.196
Morris SM Jr (2006) Arginine: beyond protein. The American Journal of Clinical Nutrition 83(2):508s–512s. https://doi.org/10.1093/ajcn/83.2.508S
Li Y, Xiao W, Luo W, Zeng C, Deng Z, Ren W, Wu G, Lei G (2016) Alterations of amino acid metabolism in osteoarthritis: its implications for nutrition and health. Amino Acids 48(4):907–914. https://doi.org/10.1007/s00726-015-2168-x
Ohnishi A, Osaki T, Matahira Y, Tsuka T, Imagawa T, Okamoto Y, Minami S (2013) Correlation of plasma amino acid concentrations and chondroprotective effects of glucosamine and fish collagen peptide on the development of osteoarthritis. J Vet Med Sci 75(4):497–502. https://doi.org/10.1292/jvms.12-0241
Tootsi K, Vilba K, Märtson A, Kals J, Paapstel K, Zilmer M (2020) Metabolomic signature of amino acids, biogenic amines and lipids in blood serum of patients with severe osteoarthritis. Metabolites 10(8). https://doi.org/10.3390/metabo10080323
Rockel JS, Kapoor M (2018) The metabolome and osteoarthritis: possible contributions to symptoms and pathology. Metabolites 8(4). https://doi.org/10.3390/metabo8040092
Wehling-Henricks M, Jordan MC, Gotoh T, Grody WW, Roos KP, Tidball JG (2010) Arginine metabolism by macrophages promotes cardiac and muscle fibrosis in mdx muscular dystrophy. PLoS One 5(5):e10763. https://doi.org/10.1371/journal.pone.0010763
Abramson SB, Amin AR, Clancy RM, Attur M (2001) The role of nitric oxide in tissue destruction. Best Pract Res Clin Rheumatol 15(5):831–845. https://doi.org/10.1053/berh.2001.0196
Xia W, Szomor Z, Wang Y, Murrell GA (2006) Nitric oxide enhances collagen synthesis in cultured human tendon cells. Journal of orthopaedic research: official publication of the Orthopaedic Research Society 24(2):159–172. https://doi.org/10.1002/jor.20060
Abramson SB (2008) Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Research & Therapy 10(Suppl 2):S2. https://doi.org/10.1186/ar2463
Kosinska MK, Liebisch G, Lochnit G, Wilhelm J, Klein H, Kaesser U, Lasczkowski G, Rickert M, Schmitz G, Steinmeyer J (2013) A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum 65(9):2323–2333. https://doi.org/10.1002/art.38053
Pousinis P, Gowler PRW, Burston JJ, Ortori CA, Chapman V, Barrett DA (2020) Lipidomic identification of plasma lipids associated with pain behaviour and pathology in a mouse model of osteoarthritis. Metabolomics: official journal of the Metabolomic Society 16(3):32. https://doi.org/10.1007/s11306-020-01652-8
Murakami M, Nakatani Y, Atsumi GI, Inoue K, Kudo I (2017) Regulatory functions of phospholipase A2. Crit Rev Immunol 37(2–6):121–179. https://doi.org/10.1615/CritRevImmunol.v37.i2-6.20
Chen Y, Crawford RW, Oloyede A (2007) Unsaturated phosphatidylcholines lining on the surface of cartilage and its possible physiological roles. J Orthop Surg Res 2:14. https://doi.org/10.1186/1749-799x-2-14
Tokumura A, Majima E, Kariya Y, Tominaga K, Kogure K, Yasuda K, Fukuzawa K (2002) Identification of human plasma lysophospholipase D, a lysophosphatidic acid-producing enzyme, as autotaxin, a multifunctional phosphodiesterase. J Biol Chem 277(42):39436–39442. https://doi.org/10.1074/jbc.M205623200
Mabey T, Taleongpong P, Udomsinprasert W, Jirathanathornnukul N, Honsawek S (2015) Plasma and synovial fluid autotaxin correlate with severity in knee osteoarthritis. Clinica chimica acta; international journal of clinical chemistry 444:72–77. https://doi.org/10.1016/j.cca.2015.01.032
Uchida H, Nagai J, Ueda H (2014) Lysophosphatidic acid and its receptors LPA1 and LPA3 mediate paclitaxel-induced neuropathic pain in mice. Mol Pain 10:71. https://doi.org/10.1186/1744-8069-10-71
Gustin C, Van Steenbrugge M, Raes M (2008) LPA modulates monocyte migration directly and via LPA-stimulated endothelial cells. American journal of physiology Cell physiology 295(4):C905–C914. https://doi.org/10.1152/ajpcell.00544.2007
Zhai G, Randell EW, Rahman P (2018) Metabolomics of osteoarthritis: emerging novel markers and their potential clinical utility. Rheumatology (Oxford, England) 57(12):2087–2095. https://doi.org/10.1093/rheumatology/kex497
Harris RC, Tallon MJ, Dunnett M, Boobis L, Coakley J, Kim HJ, Fallowfield JL, Hill CA, Sale C, Wise JA (2006) The absorption of orally supplied beta-alanine and its effect on muscle carnosine synthesis in human vastus lateralis. Amino Acids 30(3):279–289. https://doi.org/10.1007/s00726-006-0299-9
Chen R, Han S, Liu X, Wang K, Zhou Y, Yang C, Zhang X (2018) Perturbations in amino acids and metabolic pathways in osteoarthritis patients determined by targeted metabolomics analysis. J Chromatogr B Anal Technol Biomed Life Sci 1085:54–62. https://doi.org/10.1016/j.jchromb.2018.03.047
Anderson JR, Phelan MM, Foddy L, Clegg PD, Peffers MJ (2020) Ex vivo equine cartilage explant osteoarthritis model: a metabolomics and proteomics study. J Proteome Res 19(9):3652–3667. https://doi.org/10.1021/acs.jproteome.0c00143
Jiang H, Liu J, Qin XJ, Chen YY, Gao JR, Meng M, Wang Y, Wang T (2018) Gas chromatography-time of flight/mass spectrometry-based metabonomics of changes in the urinary metabolic profile in osteoarthritic rats. Experimental and therapeutic medicine 15(3):2777–2785. https://doi.org/10.3892/etm.2018.5788
Millward-Sadler SJ, Wright MO, Flatman PW, Salter DM (2004) ATP in the mechanotransduction pathway of normal human chondrocytes. Biorheology 41(3–4):567–575
Quesnele JJ, Laframboise MA, Wong JJ, Kim P, Wells GD (2014) The effects of beta-alanine supplementation on performance: a systematic review of the literature. International journal of sport nutrition and exercise metabolism 24(1):14–27. https://doi.org/10.1123/ijsnem.2013-0007
Ikeda T, Jinno T, Masuda T, Aizawa J, Ninomiya K, Suzuki K, Hirakawa K (2018) Effect of exercise therapy combined with branched-chain amino acid supplementation on muscle strengthening in persons with osteoarthritis. Hong Kong physiotherapy journal: official publication of the Hong Kong Physiotherapy Association Limited = Wu li chih liao 38(1):23–31. https://doi.org/10.1142/s1013702518500038
Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR (2005) Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr 82(5):1065–1073. https://doi.org/10.1093/ajcn/82.5.1065
Lin CC, Tsai WC, Chen JY, Li YH, Lin LJ, Chen JH (2008) Supplements of L-arginine attenuate the effects of high-fat meal on endothelial function and oxidative stress. Int J Cardiol 127(3):337–341. https://doi.org/10.1016/j.ijcard.2007.06.013
Siasos G, Tousoulis D, Vlachopoulos C, Antoniades C, Stefanadi E, Ioakeimidis N, Andreou I, Zisimos K, Papavassiliou AG, Stefanadis C (2008) Short-term treatment with L-arginine prevents the smoking-induced impairment of endothelial function and vascular elastic properties in young individuals. Int J Cardiol 126(3):394–399. https://doi.org/10.1016/j.ijcard.2007.04.057
Hristina K, Langerholc T, Trapecar M (2014) Novel metabolic roles of L-arginine in body energy metabolism and possible clinical applications. J Nutr Health Aging 18(2):213–218. https://doi.org/10.1007/s12603-014-0015-5
Fu WJ, Haynes TE, Kohli R, Hu J, Shi W, Spencer TE, Carroll RJ, Meininger CJ, Wu G (2005) Dietary L-arginine supplementation reduces fat mass in Zucker diabetic fatty rats. J Nutr 135(4):714–721. https://doi.org/10.1093/jn/135.4.714
Funding
This work was supported by the National Natural Science Foundation of China (81871848).
Author information
Authors and Affiliations
Contributions
All authors discuss the concept of the manuscript. JTL wrote the manuscript. GXN conceived the study; ZN, ZPY, and TL prepared some materials. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, JT., Zeng, N., Yan, ZP. et al. A review of applications of metabolomics in osteoarthritis. Clin Rheumatol 40, 2569–2579 (2021). https://doi.org/10.1007/s10067-020-05511-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-020-05511-8